"This paper presents a molecular motor that exhibits autonomous unidirectional rotation about a C-C single bond, driven by a cyclic redox reaction network involving concurrent biocatalytic oxidation and chemical reduction."
"The motor operates by consuming simple fuels such as oxygen and borane to facilitate rotary motion, with the directionality determined by the enantioselectivity of the associated biocatalytic processes."
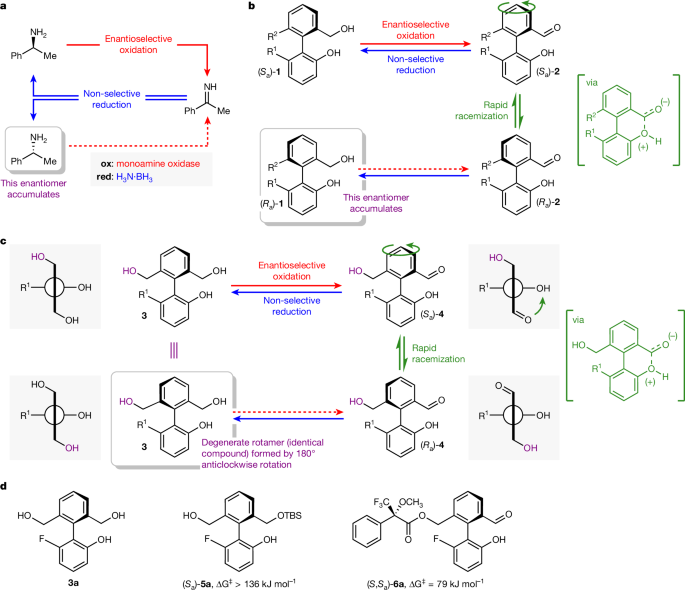
"Cyclic deracemizations are a foundational concept in synthetic chemistry that not only support the enrichment of enantiomers from a racemic mixture, but also enable new methodologies for achieving directional motion in molecular motors."
"The exploration into cyclic redox reaction networks reveals a new paradigm that seeks to construct efficient synthetic molecular motors capable of unidirectional movement, integrating concepts from both natural biocatalytic processes and synthetic chemistry."
A molecular motor has been developed that achieves autonomous unidirectional rotation around a C-C single bond. This motion is facilitated by a cyclic redox reaction network, which operates through concurrent biocatalytic oxidation and chemical reduction reactions. These reactions utilize simple fuels like oxygen and borane. Directionality of the rotary motion is determined by the enantioselectivity associated with the biocatalytic oxidation. The motor builds upon the concept of cyclic deracemization, which allows for the enrichment of one enantiomer from a racemic mixture using dissipative cyclic reaction networks.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]