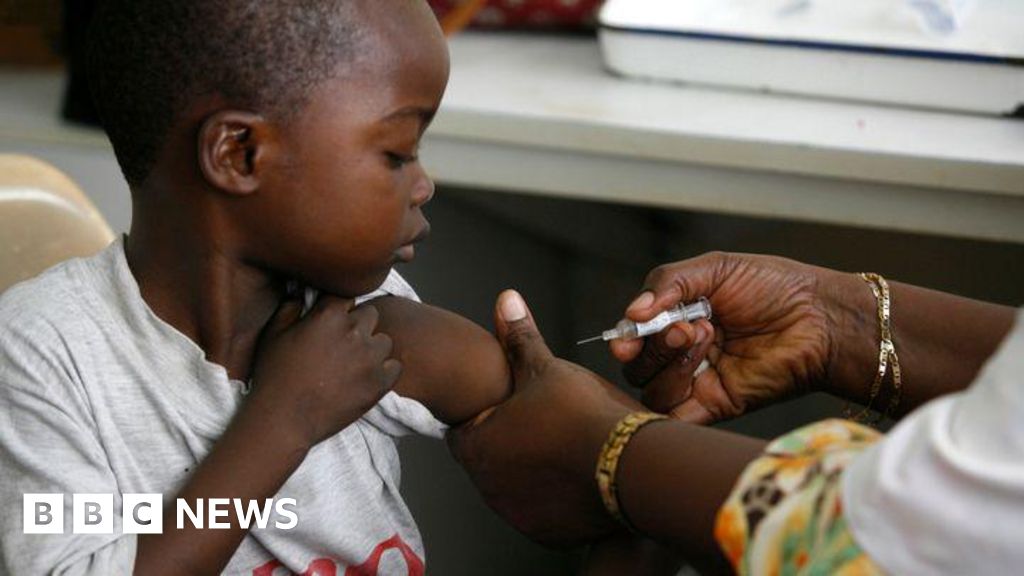
"The first malaria treatment suitable for babies and very young children has been approved for use, addressing a critical need for safer malaria treatment."
"The approval of this drug is expected to be rolled out in African countries within weeks, targeting regions with the highest rates of malaria."
"Previously, there were no approved malaria drugs formulated specifically for babies, posing overdose risks when using medications designed for older children."
"The new medication, Coartem Baby, was developed by Novartis and will be introduced on a largely not-for-profit basis, marking a significant advancement."
The first malaria treatment specifically designed for babies and very young children has received approval, with plans for rollout in African countries in the coming weeks. Prior to this, there were no approved malaria medications for this age group, leading to treatment using drugs intended for older children, which increased the risk of overdose. The newly approved drug, developed by Novartis, aims to address this significant healthcare gap, ensuring that vulnerable infants can receive appropriate treatment for malaria while minimizing risks. The initiative will be implemented on a largely not-for-profit basis.
Read at www.bbc.com
Unable to calculate read time
Collection
[
|
...
]