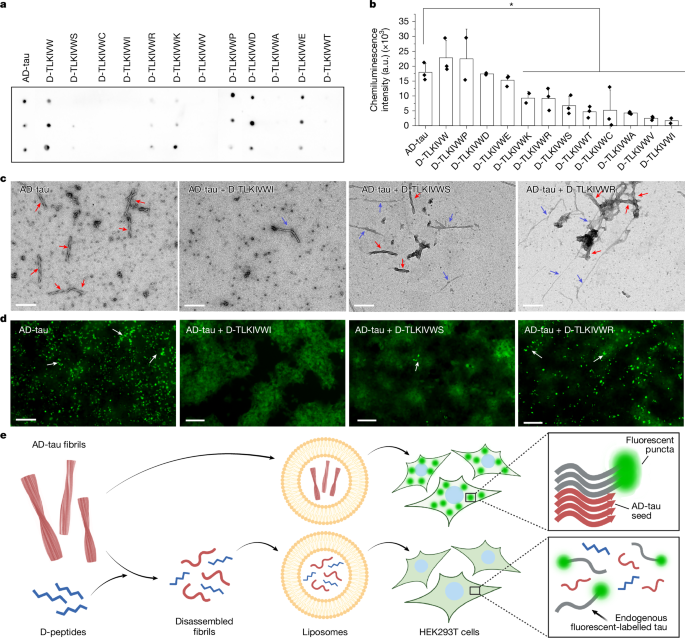
"Reducing fibrous aggregates of the protein tau is a possible strategy for halting the progression of Alzheimer's disease (AD). The D-peptide D-TLKIVWC disassembles ultra-stable tau fibrils extracted from autopsied brains of individuals with AD into benign segments without any energy source except ambient thermal agitation."
"The assembly of D-peptides into amyloid-like fibrils is essential for AD-tau disassembly. These mock-amyloid fibrils have a right-handed twist but adopt a left-handed twist when templated in complex with AD-tau."
"The release of strain accompanying conversion from left-twisted to right-twisted, relaxed mock-amyloid produces sufficient torque to break hydrogen bonds between tau molecules, leading to fragmentation."
"Understanding the mechanism and energy source of D-peptide-mediated disassembly is essential to consider it as a potential therapeutic route for Alzheimer’s disease."
D-peptide D-TLKIVWC can disassemble tau fibrils associated with Alzheimer's disease, potentially halting disease progression. The mechanism relies on D-peptide assembly into amyloid-like fibrils, which are crucial for the disassembly. Under specific conditions, these fibrils twist and convert, creating torque that disrupts the hydrogen bonds in tau aggregates, leading to their fragmentation. This process occurs under thermal agitation, making it a unique potential therapeutic approach for Alzheimer's disease due to its energy efficiency. A deeper understanding of this process is essential for further development as a treatment strategy.
Read at www.nature.com
Unable to calculate read time
Collection
[
|
...
]