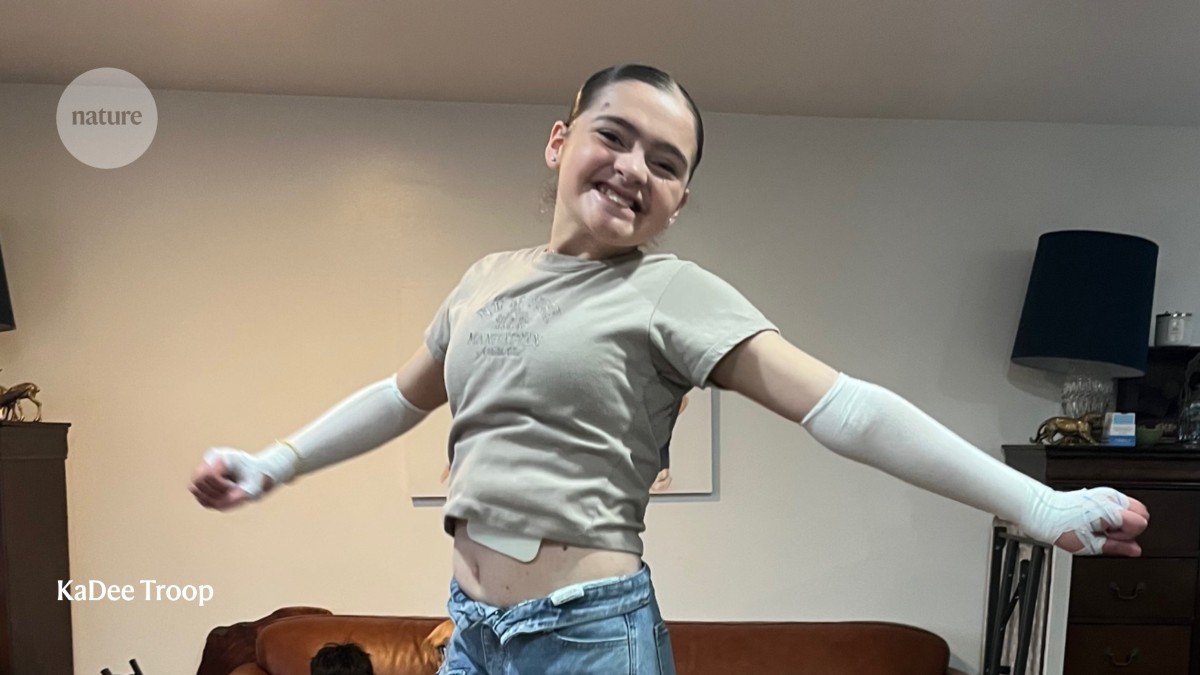
"KaDee Troop is the mother of seven adopted children, four of whom have a rare genetic disorder that causes their skin to blister and tear at the slightest touch. Wounds heal slowly - if at all - and with each re-injury, the skin becomes thinner and more fragile. For most of the children's lives, hope came in the form of gauze and bandages. That is, until 2023, when it arrived in a bottle."
"That year, US regulators approved the first gene therapy for a form of epidermolysis bullosa, the condition that affects the Troop children and is sometimes referred to as butterfly skin. All four children began applying the gel-based treatment to their raw, vulnerable lesions; the results were profound. Wounds that had stubbornly resisted healing for years began to close."
"The treatment, beremagene geperpavec (B-VEC), became the first gene-replacement therapy approved for a non-cancerous skin disorder, and the first topical gene therapy for at-home use for any disease. What's more, the success of B-VEC - developed by Krystal Biotech in Pittsburgh, Pennsylvania - proved that gene therapy could work on the skin's surface. And it has paved the way for other therapeutic strategies, including the first gene therapy delivered through skin grafts,"
Four children with epidermolysis bullosa experienced profound wound healing after starting topical beremagene geperpavec (B-VEC) following regulatory approval in 2023. The gel-based gene-replacement therapy closed long-standing lesions and restored skin resilience, enabling increased mobility, independent wound care, and use of prosthetics. B-VEC became the first gene-replacement therapy approved for a non-cancerous skin disorder and the first topical gene therapy for at-home use. The therapy's success demonstrated that gene transfer can be effective at the skin surface. The clinical proof of concept accelerated development of alternative approaches, including a recently approved gene therapy delivered via skin grafts for RDEB.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]