"Although specific bacterial taxa have been associated with favourable clinical responses to immune checkpoint blockade (ICB) in cancer patients12,13,18,19,20,21,22, the mechanisms by which the intestinal microbiota influences anti-tumour immune responses remain poorly defined. Products of the microbiota, including metabolites23,24,25 and innate receptor ligands26, may reprogramme myeloid cells27, lowering the activation threshold for antigen presentation and thereby facilitating priming and activation of tumour-reactive T cells."
"Alternatively, T cells that recognize antigens shared between commensals (microbial-associated antigens (MAAs)) and tumours (tumour-associated antigens (TAAs)) may become activated in the setting of ICB, thus enhancing anti-tumour immune responses. Because the gut microbiome encodes an enormous antigenic repertoire, commensal-derived antigens can elicit T cell responses that, in some cases, cross-react with tumour epitopes-a plausible mechanism for commensal-driven tumour control28."
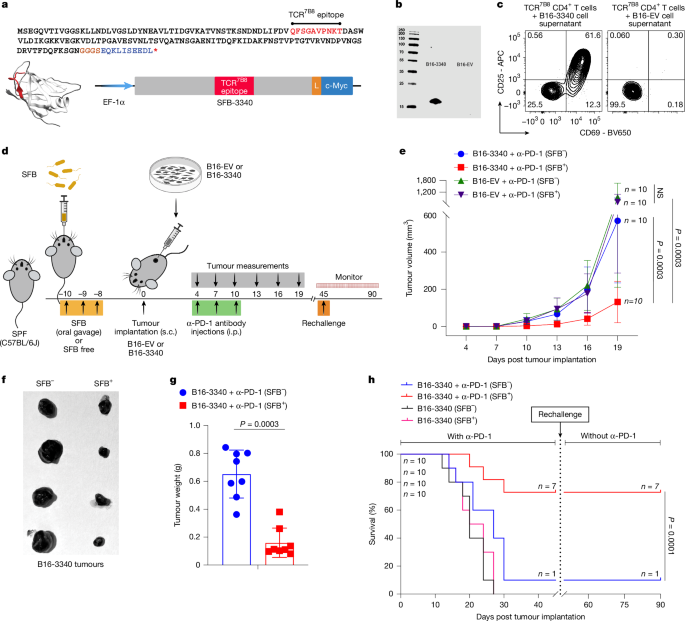
"An immunization model with skin-associated Staphylococcus epidermidis engineered to express antigens shared with implanted tumours was shown to elicit effective anti-tumour responses30, but the ability of gut commensals that elicit stereotyped T cell responses to program anti-tumour immunity has not been explored. Here we studied how a small intestine-resident commensal microbe, SFB, which induces a regulatory-like T helper 17 (T H17) cell response that enhances intestinal barrier integrity31,32, influences efficacy of ICB in controlling growth of distal tumours that share antigen with the bacterium."
Small intestine-resident segmented filamentous bacteria (SFB) induce regulatory-like T helper 17 (TH17) cells that enhance intestinal barrier integrity. SFB-primed TH17 cells can recognize antigens shared between commensals and tumours, enabling cross-reactivity with tumour epitopes and potential antigenic mimicry. Cross-reactive T cell activation can enhance anti-tumour immune responses, particularly during immune checkpoint blockade (ICB). Microbiota products such as metabolites and innate receptor ligands can reprogramme myeloid cells to lower thresholds for antigen presentation, facilitating tumour-reactive T cell priming and activation. Gut commensals with stereotyped T cell responses can program anti-tumour immunity when tumours share antigens with the commensal.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]