Science
fromenglish.elpais.com
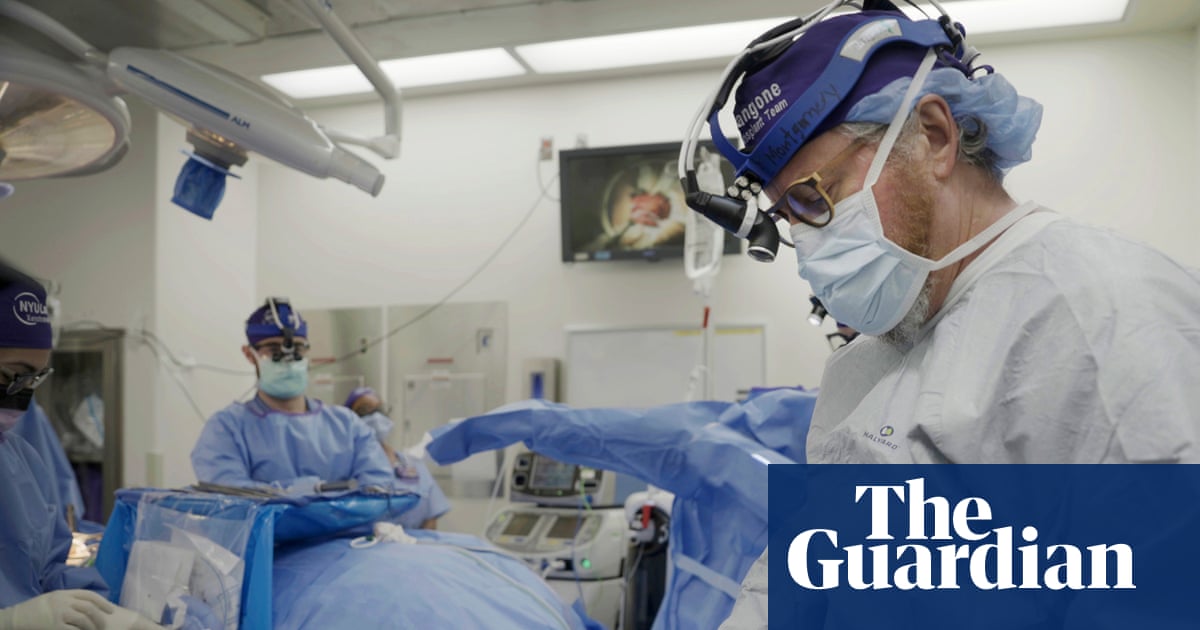
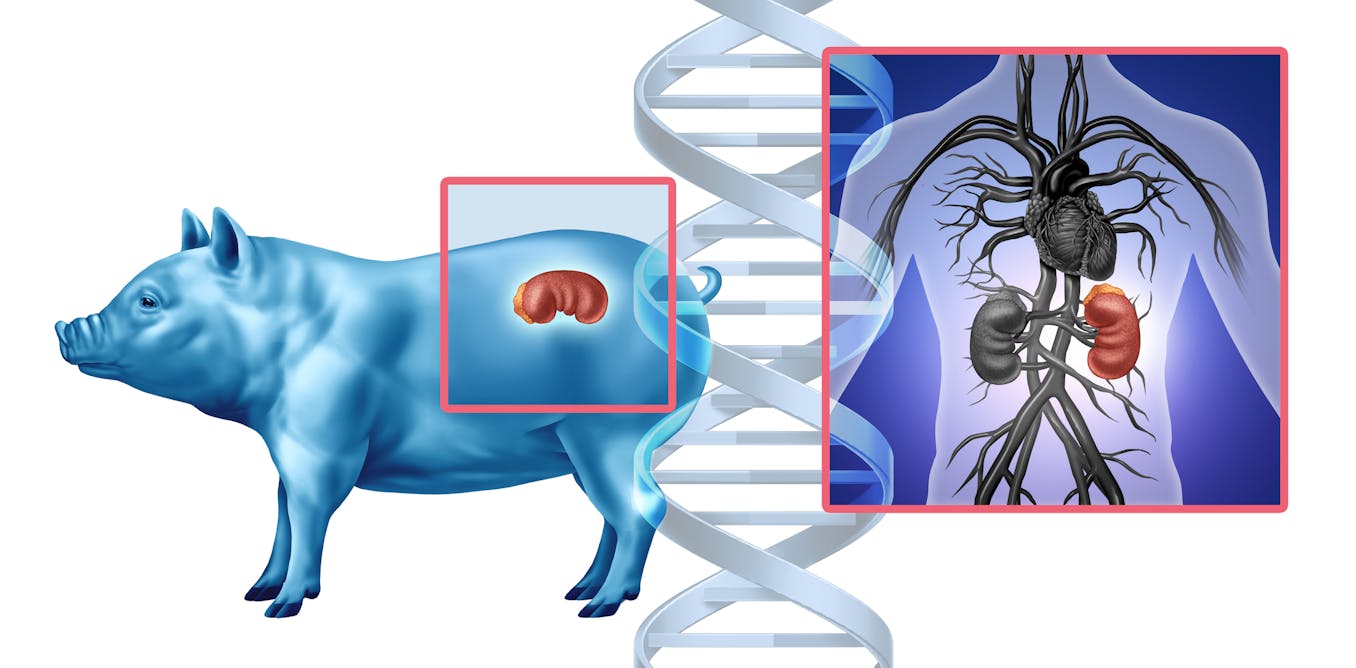
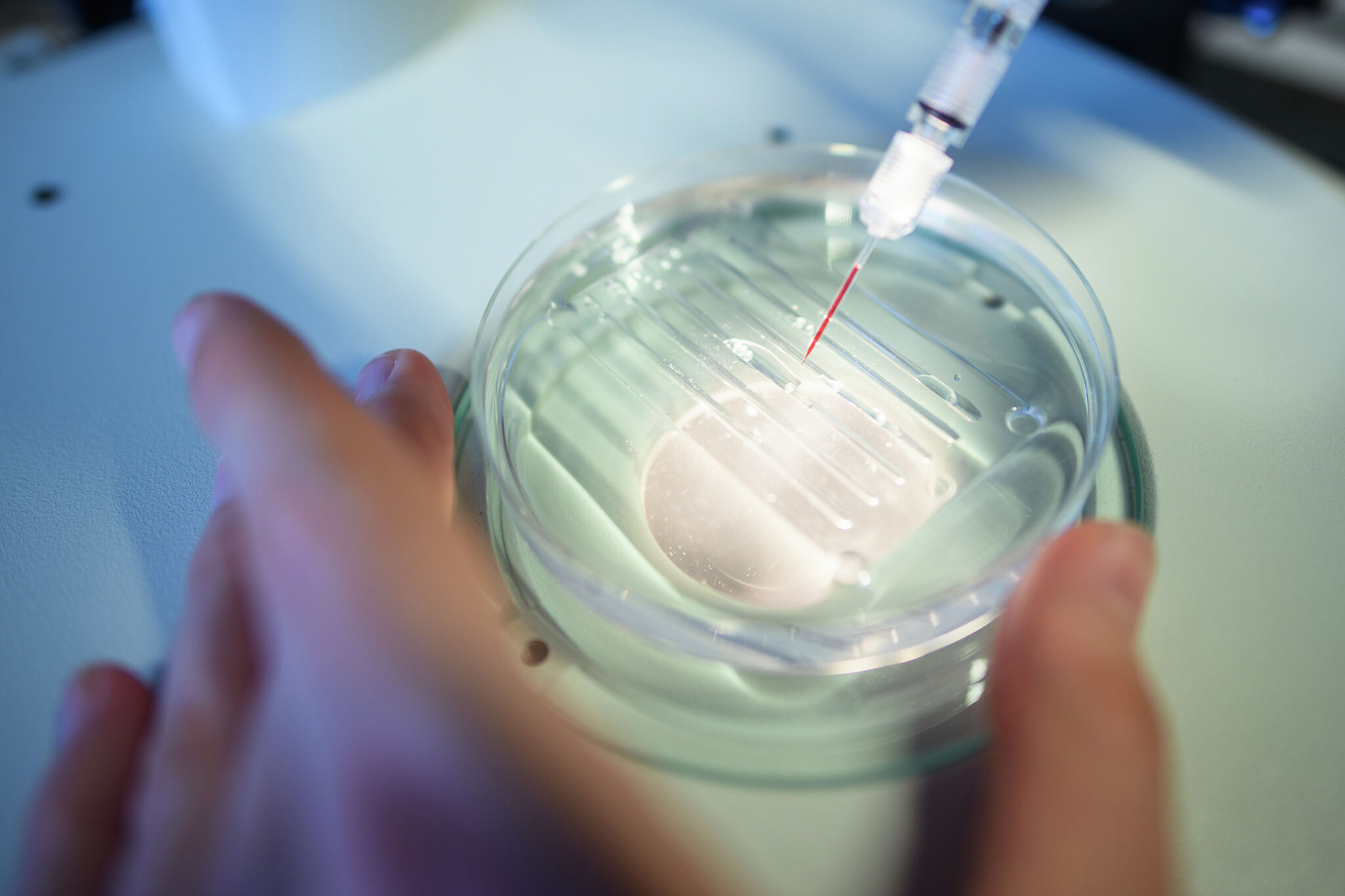
2 weeks agoEDEN: The AI system that learns from a million species to design new treatments
AI-trained evolutionary genetic models enable programmable gene editing to replace defective genetic codes and reprogram cells, enabling new therapies for cancer and hereditary diseases.