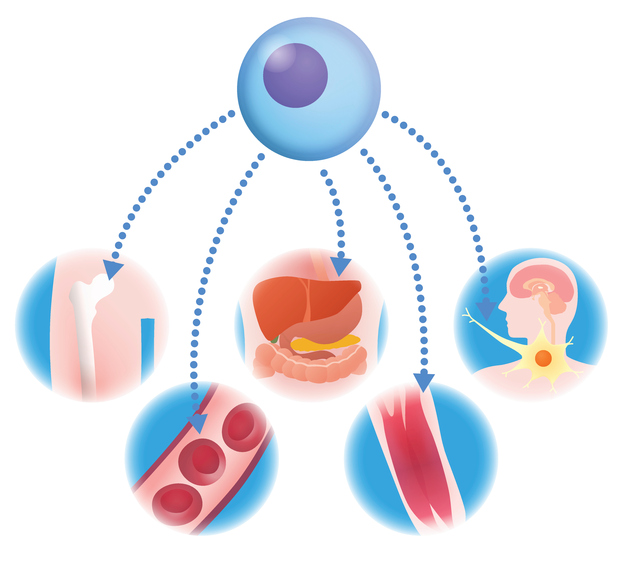
"The law allows licensed physicians in Florida to use non-FDA-approved stem cell therapies for specific indications in orthopedics, wound care, and pain management."
"Compliance requirements are stringent, ensuring that stem cells are ethically sourced and manufactured in FDA-regulated facilities, reinforcing accountability in treatment."
"Patients are expected to benefit from expanded treatment options, reflecting a growing acceptance and demand for alternatives to standard care in stem cell therapy."
"The initiative highlights the complex balance between innovation in medical treatments and the need for oversight and ethical standards in clinical practices."
On July 1, Florida enacted a law permitting licensed physicians to administer certain non-FDA-approved stem cell therapies. This aims to expand treatment options for patients dissatisfied with standard care, particularly in orthopedics, wound care, and pain management. The law enforces stringent regulatory compliance, requiring ethical sourcing of stem cells from placental perinatal sources, adherence to Good Manufacturing Practices, and manufacturing in FDA-registered facilities. The legislation seeks to navigate the intersection of clinical ethics and medical innovation while addressing the growing global demand for stem cell therapies.
Read at MedCity News
Unable to calculate read time
Collection
[
|
...
]