"Many chemicals have a handedness, or chirality, and can exist in two mirror-image forms. But the building blocks of life tend to stick to one or the other."
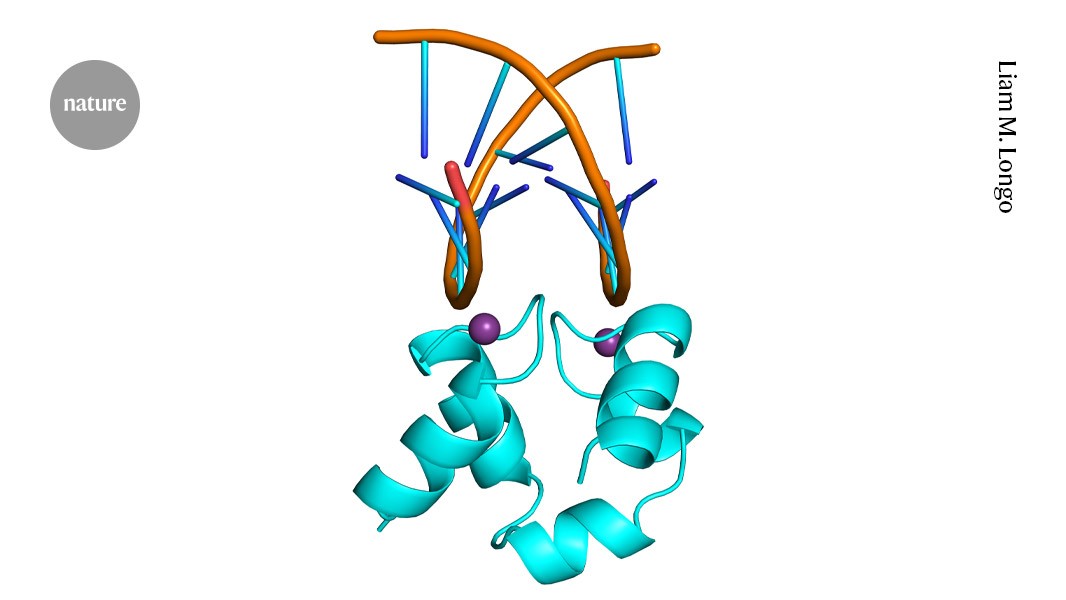
"Liam Longo, a protein historian at the Tokyo Earth-Life Science Institute, was examining a protein fragment, or peptide, that recognizes nucleic acids and is commonly found in DNA repair enzymes."
Researchers found an ancient protein with ambidextrous properties, capable of functioning in both left- and right-handed mirror-image forms. This discovery suggests the existence of a time in Earth's history when life may have relied on such mirror-image molecules. The study, led by Liam Longo at the Tokyo Earth-Life Science Institute, revealed that the symmetrical helix-hairpin-helix motif in this protein allowed it to bind nucleic acids effectively. Experiments demonstrated that both versions of the peptide had nearly equivalent binding capabilities, hinting at a deep evolutionary connection to life's early molecular structures.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]