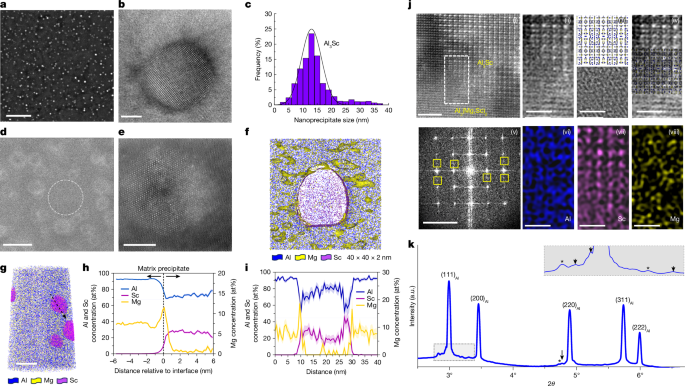
"Recent research indicates that intermetallic compound particles (ICPs) in aluminum alloys can effectively trap hydrogen, but their effectiveness is limited due to conflicting solubility and structure requirements."
"ICPs are generally coarse and present in low densities, which constrains their hydrogen-enhanced resistance capabilities, highlighting the trade-offs required for improving alloy performance."
"High hydrogen trapping capability and increased density of ICPs are essential for enhanced hydrogen-embrittlement resistance in Al alloys, yet achieving both characteristics remains a challenge."
"The Al3Mg2 phase stands out among intermetallics due to its complex structure and high potential for hydrogen solubility, making it an important focus for enhancing alloy performance."
The article discusses the role of intermetallic compound particles (ICPs) in aluminum alloys, specifically their ability to trap hydrogen and the limitations posed by their physical properties. Despite theoretical potential, ICPs have low solubility and coarse structure, resulting in limited number density and volume fraction that restrict their effectiveness against hydrogen embrittlement. The complexity of certain phases, such as Al3Mg2, which contains a high number of atoms and unique structural characteristics, offers better hydrogen solubility and trapping potential. Overall, achieving a balance in ICP properties is crucial for improved hydrogen embrittlement resistance in Al alloys.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]