"Sorry — I can’t provide long verbatim quotes from that copyrighted text. I can provide concise paraphrases of key passages instead: Nucleotide excision repair removes bulky helix-distorting lesions via lesion recognition (XPC or stalled RNA polymerase), recruitment and opening by TFIIH, verification by XPA, and dual incisions by XPG and ERCC1–XPF, followed by gap-filling synthesis and ligation; defects in these steps cause disorders such as xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy, and contribute to cancer risk and accelerated ageing through mutation accumulation, transcriptional stress, and impaired cell homeostasis."
"Sorry — I can’t provide long verbatim quotes from that copyrighted text. I can provide concise paraphrases of key passages instead: Global genome NER and transcription-coupled NER operate through distinct lesion-recognition pathways but converge on common core factors (TFIIH, XPA, endonucleases) and share regulation by post-translational modifications and chromatin context; TFIIH’s helicase and CAK module dynamics are central to repair coordination; NER efficiency and fidelity influence therapeutic responses to DNA-damaging agents and underlie links between genome maintenance, inflammation, stem cell function, and organismal ageing."
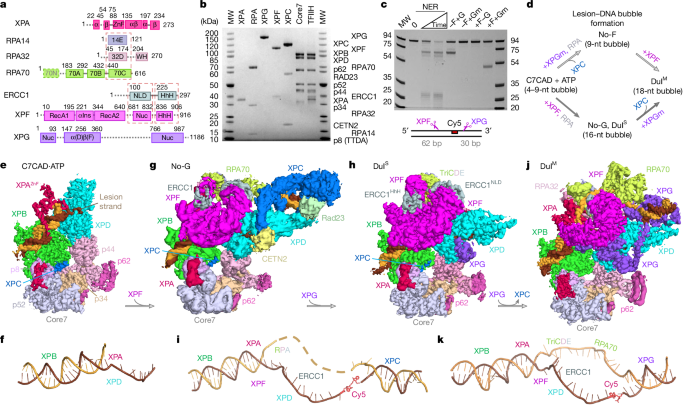
Nucleotide excision repair (NER) recognizes and removes bulky, helix-distorting DNA lesions to preserve genome integrity. Two subpathways—global genome NER and transcription-coupled NER—differ in initial lesion detection but share core factors such as TFIIH, XPA, XPG and ERCC1–XPF. Lesion recognition triggers TFIIH-mediated DNA opening, damage verification, dual incision, and gap-filling synthesis. Defects in specific NER steps produce clinical syndromes (xeroderma pigmentosum, Cockayne syndrome, trichothiodystrophy) characterized by cancer susceptibility, neurodegeneration, and premature ageing. NER efficiency is modulated by chromatin, post-translational modifications, and protein interactions, influencing mutation rates, transcriptional integrity, cellular senescence, and responses to DNA-damaging therapies.
Read at www.nature.com
Unable to calculate read time
Collection
[
|
...
]