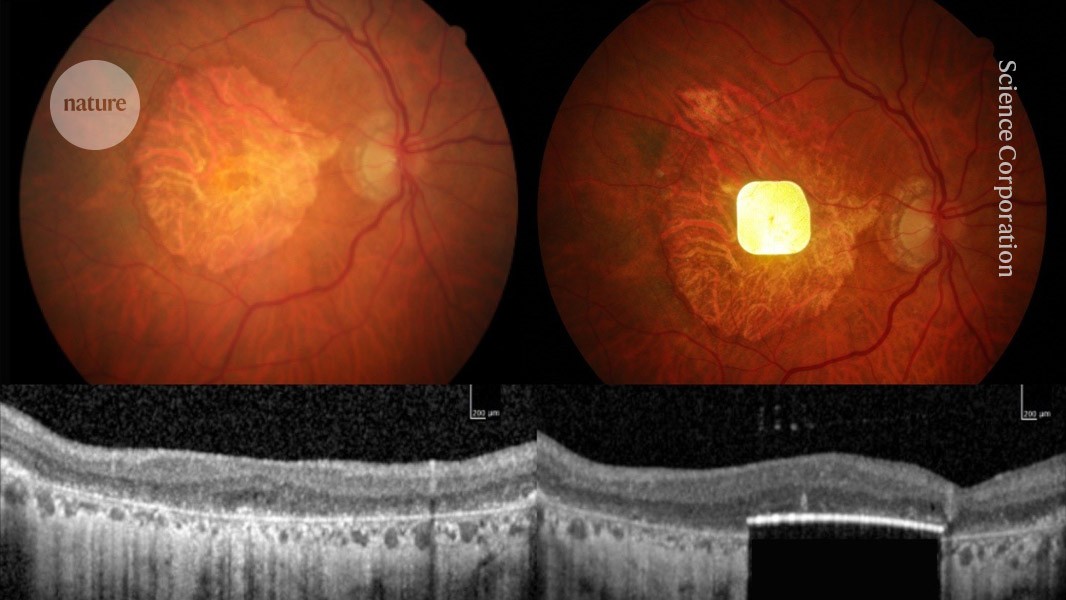
"Scientists have used an eye implant to improve the vision of dozens of people left functionally blind by age-related macular degeneration (AMD). The implant, which measures 2 millimetres by 2 millimetres, and is just 30 micrometres thick, is surgically inserted beneath the retina to replace the light-sensitive cells that have been lost to the disease."
"The clinical trial, which is described today in The New England Journal of Medicine, involved 38 people with advanced AMD whose retinas had degenerated severely. One year after device implantation, 80% of participants had gained a clinically meaningful improvement in their vision. "Where this dead retina was a complete blind spot, vision was restored," says trial leader Frank Holz, an ophthalmologist at the University of Bonn in Germany. "Patients could read letters, they could read words, and they could function in their daily life.""
"Despite some minor events related to implantation surgery, the trial's safety-monitoring board viewed the device's benefits as outweighing its risks. In June, the device's owners - the San Francisco-based neurotechnology company Science Corporation - applied for certification that would allow the device to be sold on the European market. "I think this is an exciting and significant study, which has been well-designed and analysed. It gives hope for providing vision in patients for whom this was more 'science-fiction' than reality," says Francesca Cordeiro, an ophthalmologist at Imperial College London."
An ultra-thin 2 millimetre by 2 millimetre retinal implant, 30 micrometres thick, was surgically placed beneath the retina to replace lost light-sensitive cells. A clinical trial enrolled 38 people with advanced dry age-related macular degeneration and severely degenerated retinas. One year after implantation, 80% of participants experienced clinically meaningful improvement in central vision, enabling activities such as reading letters and words and improving daily function. The safety-monitoring board judged benefits to outweigh minor surgical events. The device’s owners applied for European certification. The approach targets restoration of high-acuity central vision in millions affected by advanced dry AMD.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]