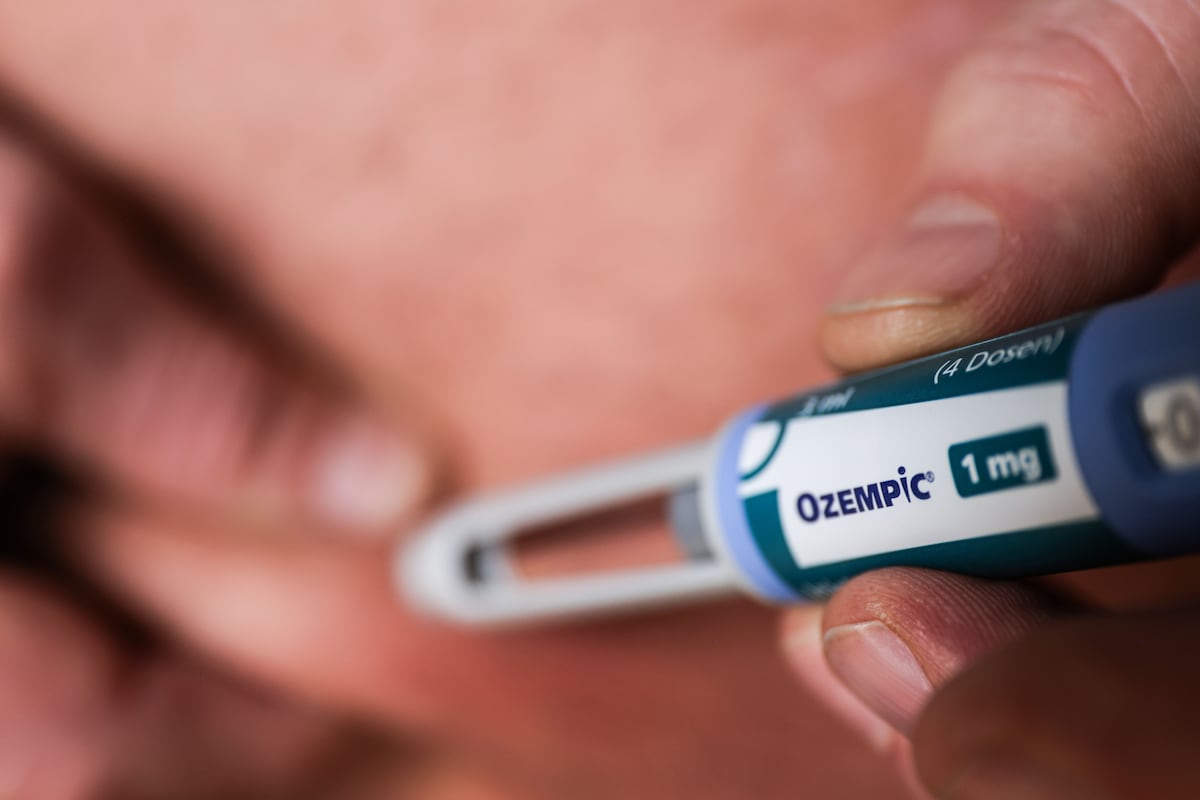
"The MHRA estimates that one-third of adverse reactions from medications could be prevented with prior genetic testing, particularly for GLP-1 drugs."
"Despite alarming reports of drug-related deaths, published information does not clearly indicate an increased risk among British users of GLP-1 medications."
"Pancreatitis is a risk documented in clinical trials, but occurred in less than 0.5% of patients using semaglutide, indicating a controlled scenario."
"Endocrinologist Cristobal Morales emphasizes the safety of GLP-1 drugs over the last 20 years, yet warns of dangers when these drugs are misused or self-prescribed."
The UK Medicines and Healthcare products Regulatory Agency is investigating genetic influences on pancreatic inflammation associated with GLP-1 drugs. They suggest that genetic testing could prevent a significant proportion of adverse reactions. Recent media reports highlighted serious concerns, yet data does not support heightened risk among users. Clinical trials have shown pancreatitis occurrences in under 0.5% of patients. Medical experts express caution regarding the misuse of these medications, particularly when procured without professional oversight, which could lead to more severe side effects than anticipated.
Read at english.elpais.com
Unable to calculate read time
Collection
[
|
...
]