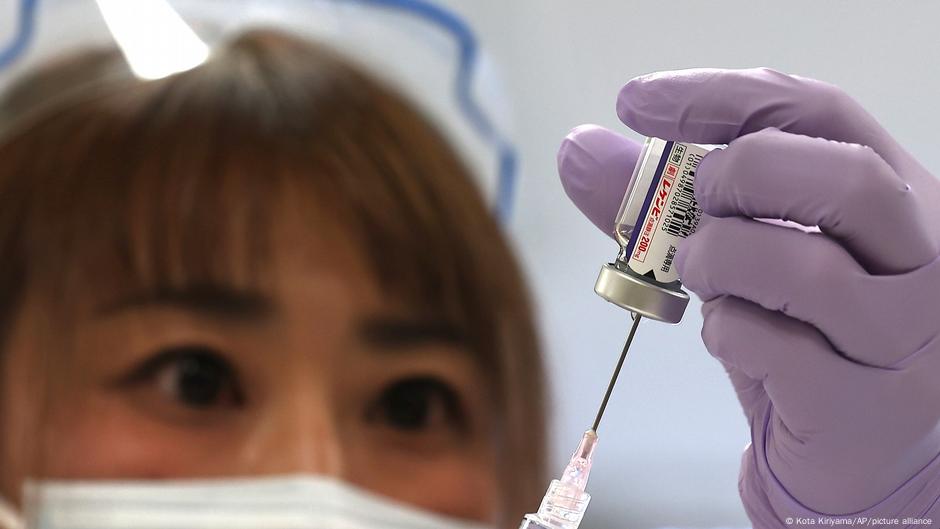
"The European Commission has approved lecanemab, the first therapy targeting the underlying processes of Alzheimer's, marking a significant advancement in the treatment landscape."
"Lecanemab, sold under the brand name Leqembi, is now authorized in the EU, following a prior recommendation by the European Medicines Agency."
The European Commission has approved lecanemab, the first Alzheimerâs treatment targeting the diseaseâs underlying processes, marking a historic milestone in EU healthcare. Intended for patients in the early stages of Alzheimer's, lecanemab represents a shift from conventional therapies focused on symptomatic relief. The approval follows a change in stance by the European Medicines Agency, which had previously hesitated. Despite its approval, experts caution that the drugâs accessibility is limited, as only a small percentage of Alzheimerâs patients meet the criteria for treatment. Lecanemab is already authorized in the US, UK, and Japan.
Read at www.dw.com
Unable to calculate read time
Collection
[
|
...
]