#gene therapy
#gene therapy
[ follow ]
fromSFGATE
2 months agoBay Area biotech company declares bankruptcy, over $10 million in debts
The biotech company, which is headquartered in Milpitas, turned in a Chapter 7 filing, meaning that it seeks liquidation, rather than reorganization. The document is so sparse that it prompted a request from the court's deputy clerk for more information. But it depicts a company in dire straits: ASC Therapeutics estimates that it has between $100,000 and $500,000 in assets and between $10 million and $50 million in liabilities.
US news
fromNature
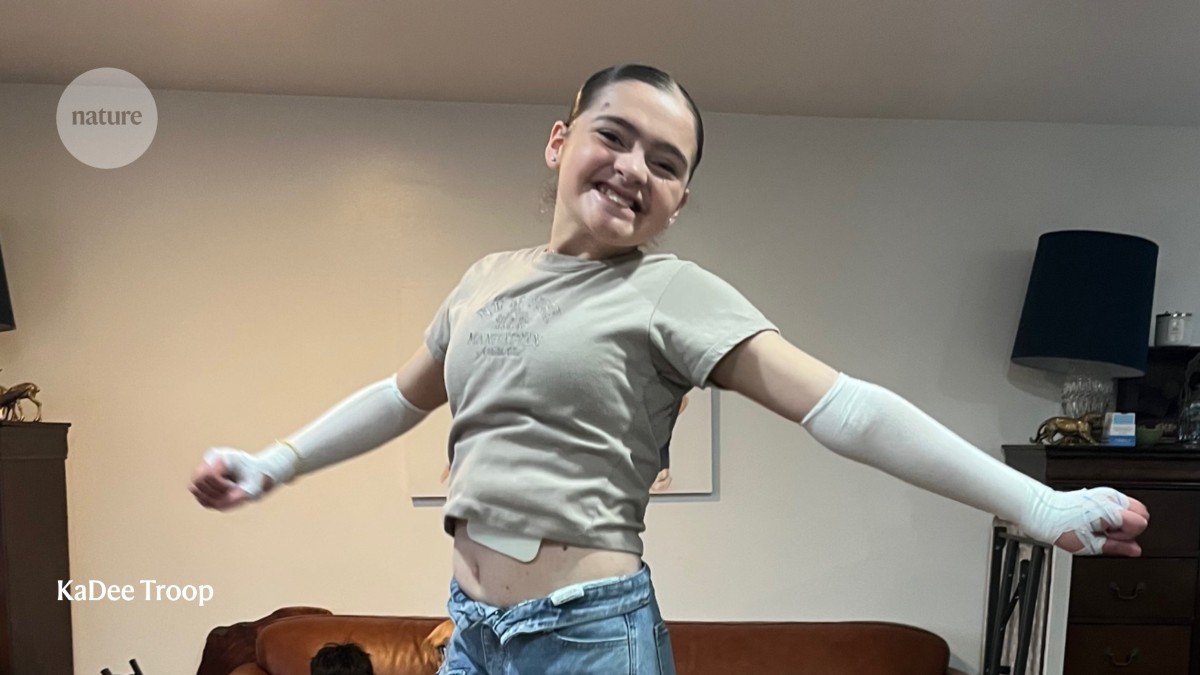
2 months agoHow to fix genetic 'nonsense': versatile gene-editing tool could tackle a host of diseases
A single multipurpose gene-editing tool can correct several genetic conditions by restoring proteins that have been truncated by disease-causing mutations. The method might one day overcome a key stumbling block faced by gene-editing therapies: the need to design a bespoke treatment for each disease. The new approach, called PERT, combines gene editing with engineered RNA molecules that allow protein synthesis to continue even when a mutation in the DNA tells it to stop prematurely.
Science
Intellectual property law
fromIPWatchdog.com | Patents & Intellectual Property Law
2 months agoChakrabarty Redux: Are Genetically Engineered Host Cells Patent Eligible?
Patent eligibility of genetically engineered host cells remains uncertain following Regenxbio v. Sarepta despite Chakrabarty’s recognition of man-made organisms as patent eligible.
fromFuturism
2 months agoScientists Say They've Figured Out a Way to Reprogram the Pancreas to Produce GLP-1s Without Ozempic
GLP-1 is the naturally produced hormone whose function is being mimicked by drugs like semaglutide and tirzepatide, the active ingredients in popular and highly effective weight-loss drugs like Wegovy and Zepbound. As CNN reports, two biotech startups are working on gene therapy treatments that would require, in a best-case scenario, a single injection that would reprogram the body's cells to produce the hormone instead of relying on weekly injections like current drugs.
Medicine
fromNature
4 months agoAncient viral DNA helps human embryos develop
Research suggests that ancient viral DNA embedded in the human genome is playing a key role in early embryo development. Around 8% of our genome consists of endogenous retrovirus DNA - the remnants of ancient infections, but knowledge of their activity is limited. Now, a team shows that these sequences are required for the correct development of lab-derived embryo analogues, and for the switching on of human-specific genes.
Science
fromNature
5 months agoSkin: preserving the health of a multi-talented organ
The word skin has come to stand for superficiality and lack of depth, but human skin defies such connotations. For one thing, it is enormous, accounting for roughly 15% of a person's body weight. Skin is also remarkable for its diverse roles: protection against pathogens, temperature regulation and, of course, sensation. Like any part of the body, skin is fallible to disease. But scientists are making great strides in protecting and improving the organ's health.
Medicine
fromwww.theguardian.com
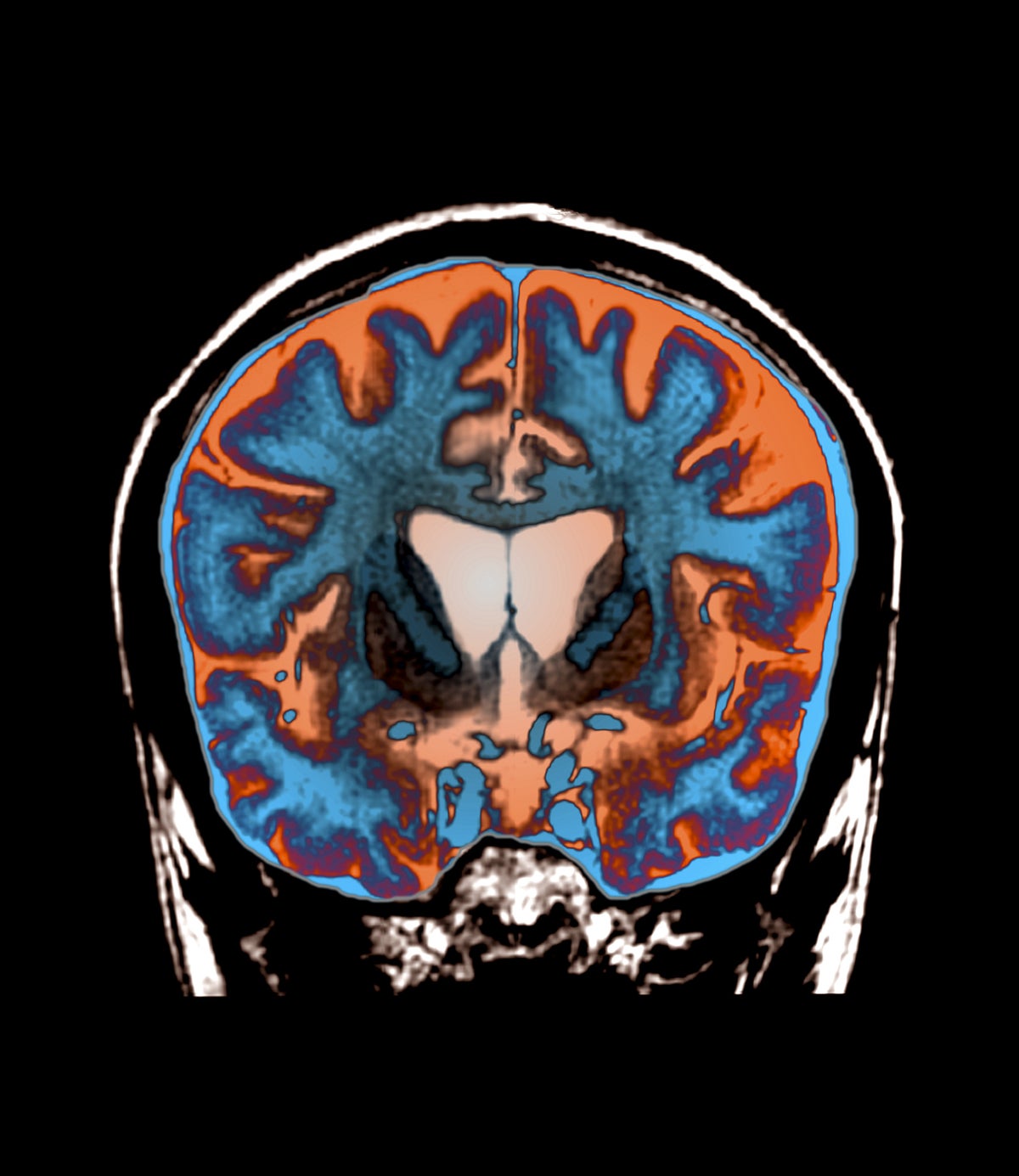
7 months agoTransformative': the UK lab working on a way to halt genetic type of dementia
"It may be one of the first dementias to have a definitive treatment, a cure if you like, a really transformative treatment that allows people to live much longer and much more normal lives..."
London startup
fromNews Center
7 months agoTaking the Science to Market: Start-ups at Feinberg - News Center
The significant discovery focused on improving the degradation of aggregation-prone proteins involved in neurodegenerative diseases, ultimately leading to a start-up company focused on developing targeted treatments.
NYC startup
fromenglish.elpais.com
8 months agoCarl H. June, creator of CAR-T therapies: The genetic medicine of the future will be able to treat everything, from diabetes to chronic infections'
The transformation in medicine driven by personalized gene therapies promises cures for many diseases, including cancer, diabetes, and autoimmune conditions, but widespread application may take decades.
Alternative medicine
[ Load more ]