Health
fromwww.npr.org
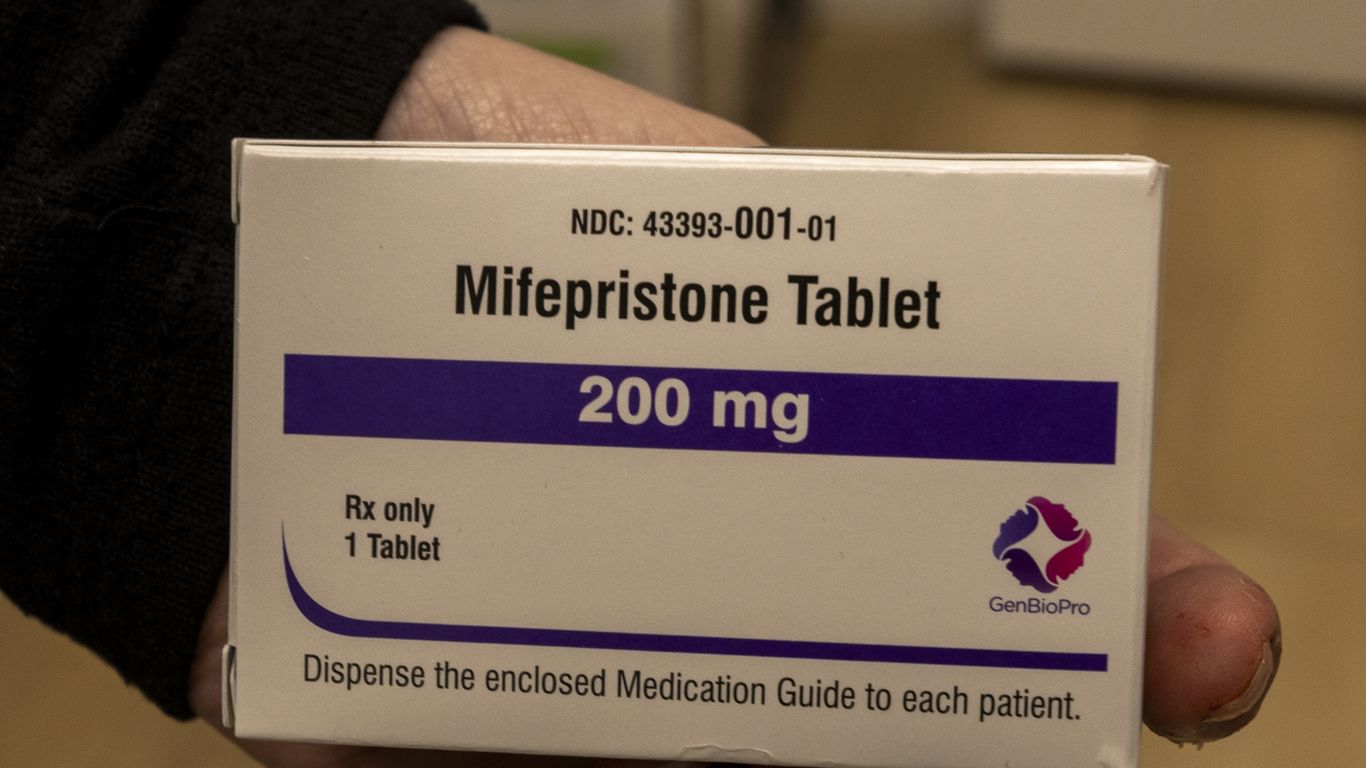
1 day agoInfluencers are promoting peptides for better health. What's the science say?
Many synthetic peptides sold online are experimental and unproven for human use, posing safety risks despite a few FDA-approved peptide medicines.