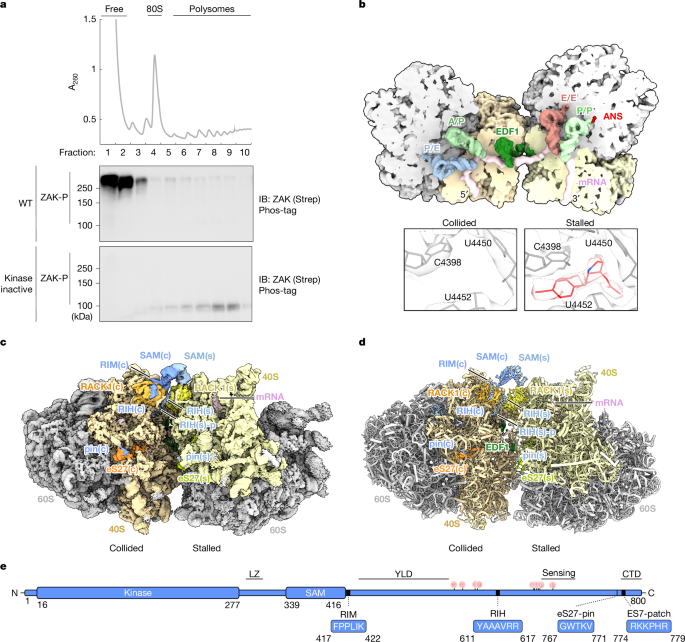
"The ribosome translates mRNA into protein, often with multiple ribosomes on a given mRNA called polysomes. Ribosomes are also essential sensors of cellular stress and can alert the cell of nutrient deprivation2, damage to mRNAs and chemical insults that directly target and damage ribosomes3,4,5. Such cellular stresses cause ribosomes to stall on problematic mRNA, resulting in the lagging ribosome colliding with the stalled ribosome. These ribosome collisions are a key signal to activate both quality control pathways and broad stress signalling responses4,6."
"For one of these signalling pathways, the ribotoxic stress response (RSR), the mitogen-activated protein kinase kinase kinase (MAP3K) ZAKα (referred to hereafter as ZAK) has a central role in orchestrating the RSR7,8,9. Previous studies have demonstrated that ZAK interacts constitutively with ribosomes during unstressed conditions but becomes activated (via autophosphorylation) and is released from the ribosome upon cellular stresses that impair translation1,10."
"Although previous studies have argued that ribosome collisions are key determinants of ZAK activation1, other studies have suggested that both colliding and individually stalled ribosomes may be potent triggers10,11. In either case, ZAK activates the stress-activated protein kinases (SAPKs) p38 and JNK, leading to cell cycle arrest and/or apoptosis12,13,14. Kinase regulation often requires scaffold proteins15, and ZAK has been shown to interact with 14-3-3 proteins off the ribosome in a canonical phosphorylation-dependent manner downstream of activation10,12."
Ribosomes translate mRNA into protein and function as sensors of cellular stress, where stalled ribosomes can cause trailing ribosomes to collide. Ribosome collisions act as signals that trigger quality control pathways and broad stress signalling responses. The MAP3K ZAKα occupies a central position in the ribotoxic stress response, interacting constitutively with ribosomes, undergoing autophosphorylation and release upon translation-impairing stresses, and activating SAPKs p38 and JNK. Both colliding and individually stalled ribosomes can trigger ZAK activation. Kinase regulation involves scaffold proteins, with RACK1 positioned at the collision interface and 14-3-3 interacting with ZAK off the ribosome.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]