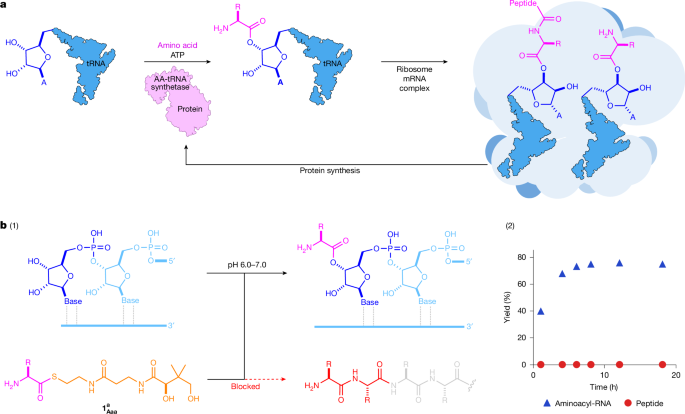
"At life's functional core, there is a complex and inseparable interplay between nucleic acids and proteins, but the origin of this relationship remains a mystery. Although nucleic acids store, replicate and transmit sequence information through their inherent structural capacity for molecular (self-)recognition6,7,8,9, proteins are the molecular, structural and catalytic workhorses of life. Unlike nucleic acids, peptides do not innately replicate in a sequence-specific manner10,11, so life must control and transmit the peptide sequences that are essential to its survival through nucleic acid encoding12."
"However, all proteins are built through the iteration of a universally conserved two-stage process, ribosomal peptide synthesis (RPS). In the first stage of RPS, transfer RNAs (tRNAs) are aminoacylated, loading an activated amino acid onto the 2′,3′-diol moiety of the tRNA, before these subsequently undergo ribosome-catalysed peptide synthesis (Fig. 1a). Notably, throughout RPS, the activated amino acids are orchestrated and controlled through their covalent attachment to RNA and so the selective formation of RNA esters underpins, and must predate, RPS."
Life depends on a molecular partnership between nucleic acids and proteins where nucleic acids store and transmit sequence information while proteins perform structural and catalytic roles. Peptides lack inherent, sequence-specific replication, so peptide sequences must be controlled and transmitted by nucleic acid encoding. The emergence of nucleotide-directed peptide biosynthesis is challenging to reconstruct given the complexity of protein synthesis. All proteins arise via a conserved two-stage ribosomal peptide synthesis pathway in which tRNAs are first aminoacylated, placing activated amino acids on their 2′,3′-diol moieties, and then undergo ribosome-catalysed peptide assembly. Selective formation of RNA esters underpins and must precede this pathway.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]