"This early stage research could one day also be used to treat infertility for women of advanced maternal age "or those who are unable to produce viable eggs due to previous treatment of cancer or other causes," according to an OHSU post. Yes, but: The Portland-based team noted several limitations in their proof-of-concept study, published in Nature Communications on Tuesday, notably that all of the embryos had chromosomal abnormalities."
"And only 9% of the 82 eggs created in the Oregon lab developed six days after fertilization, when embryos are typically transferred to establish a pregnancy through IVF. None were cultured beyond that point. Still, senior study author Shoukhrat Mitalipov, director of the OHSU Center for Embryonic Cell and Gene Therapy, noted researchers have "achieved something that was thought to be impossible.""
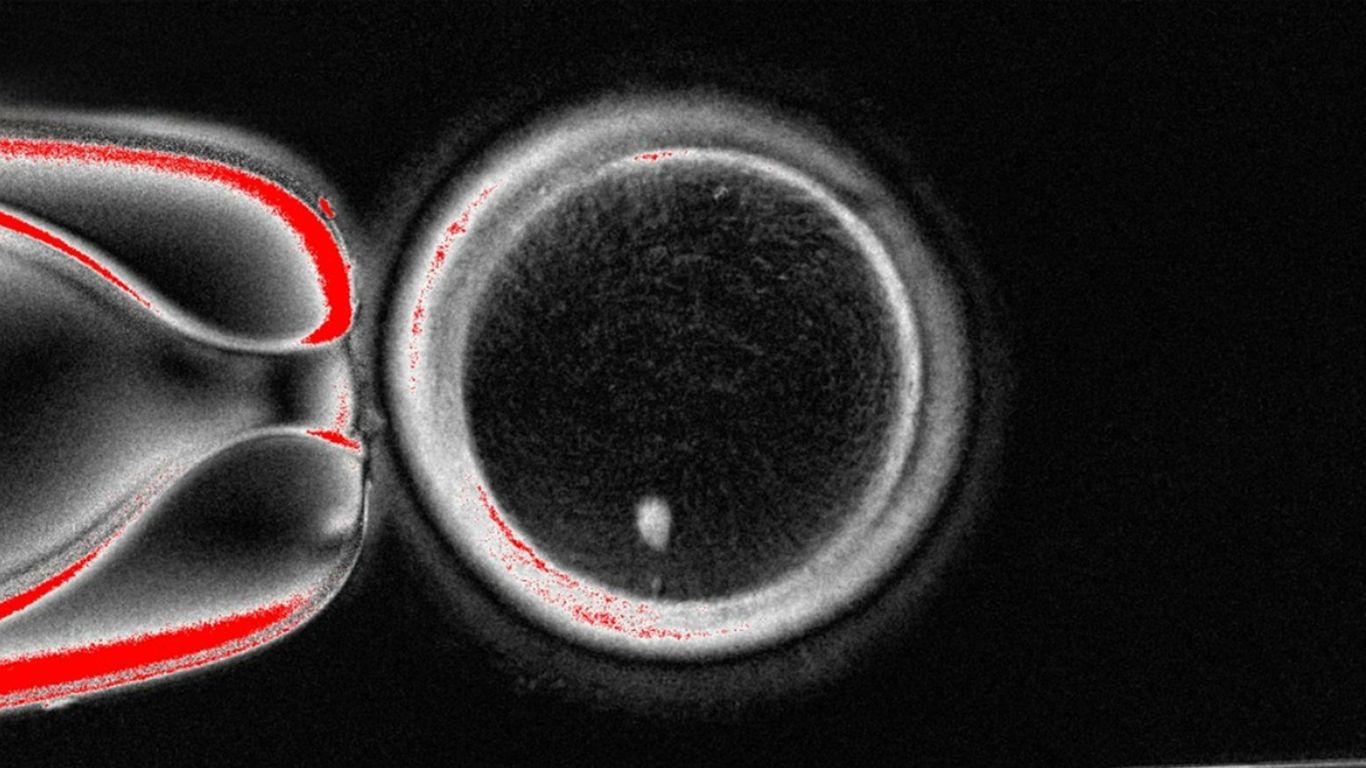
"What they did: The early stage research involved a combination of cloning and fertilization to isolate the nucleus of a skin cell and implant it into a donor egg stripped of its nucleus, a processed pioneered in the cloning of Dolly the sheep in 1997. While Dolly was created after the cloning of one parent, the researchers' work resulted in embryos with chromosomes contributed from both parents."
Researchers combined cloning and fertilization to transfer a skin-cell nucleus into an enucleated donor egg, producing embryos carrying chromosomes from both parents. The process, called mitomeiosis, activated eggs and induced halving of chromosomes to mimic natural gamete formation. Of 82 reconstructed eggs, 9% developed to six days after fertilization, the typical embryo-transfer stage in IVF; none were cultured past that point. All embryos exhibited chromosomal abnormalities. The approach could potentially enable creation of egg- or sperm-like cells to address infertility in women of advanced maternal age or those rendered unable to produce viable eggs by prior cancer treatments.
Read at Axios
Unable to calculate read time
Collection
[
|
...
]