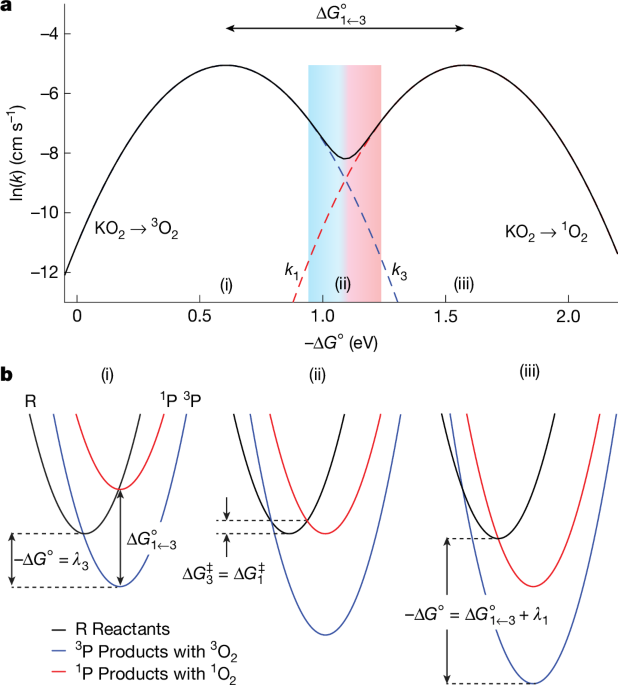
"However, the factors determining the formation of singlet oxygen, rather than the relatively unreactive triplet oxygen, are unknown. Here we report that the release of triplet or singlet oxygen is governed by individual Marcus normal and inverted region behaviour. We found that as the driving force for the reaction increases, the initially dominant evolution of triplet oxygen slows down, and singlet oxygen evolution becomes predominant with higher maximum kinetics."
"This behaviour also applies to the widely observed superoxide disproportionation, in which one superoxide is oxidized by another, in both non-aqueous and aqueous systems, with Lewis and Brønsted acidity controlling the driving forces. Singlet oxygen yields governed by these conditions are relevant, for example, in batteries or cellular organelles in which superoxide forms. Our findings suggest ways to understand and control spin states and kinetics in oxygen redox chemistry, with implications for fields, including life sciences, pure chemistry and energy storage."
Release of triplet or singlet oxygen follows Marcus normal and inverted region behavior, so kinetic rates vary with reaction driving force. At low to moderate driving force, triplet oxygen evolution dominates and increases with driving force; beyond a maximum, triplet evolution slows in the inverted region while singlet oxygen evolution accelerates and reaches higher maximum kinetics. The same Marcus-controlled switching applies to superoxide disproportionation reactions in both non-aqueous and aqueous media. Lewis and Brønsted acidity modulate the driving forces and therefore control singlet oxygen yields. Control over these parameters enables manipulation of spin states and reaction kinetics in batteries, biological organelles, and other oxygen redox systems.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]