"In the bulk and at room temperature (RT), water exhibits exceptionally high dielectric constant ( εbulk ≈ 80) and high (for a wide-bandgap insulator) electrical conductivity ( σbulk ≈ 10 −5 S m −1)1. Both characteristics are inherently connected with the ability of water molecules to form hydrogen bonds11,12,13 and are key to the main properties of water. Among them is its remarkable ability to dissolve more substances than any other liquid7,8, which originates from the high εbulk of water that efficiently suppresses Coulomb interactions between solutes."
"Within these layers, the hydrogen-bond network is greatly altered compared with that of bulk water, no longer following the ice rules14. Accordingly, the electrical properties of water near surfaces and inside nanoscale cavities are expected to be different from those of bulk water2,3. These differences have been the subject of intense research. In particular, proton conductivity of near-surface water has been reported to become much larger than σbulk, although the magnitude of this enhancement and the underlying mechanism remain debated15,16,17,18,19."
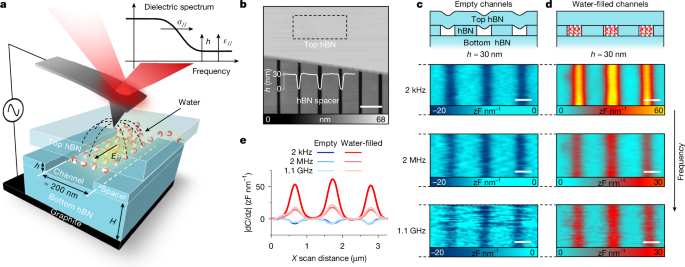
Water at bulk and room temperature has a high dielectric constant (~80) and relatively high electrical conductivity (~10^-5 S m^-1) due to hydrogen-bond networks. High dielectric screening enables exceptional solvation and underpins biochemical processes such as protein folding, nucleic acid interactions, and ion transport. At solid interfaces, liquid water forms distinct layered structures with altered hydrogen bonding that violates ice rules. Electrical properties near surfaces and in nanoscale cavities therefore differ from bulk values. Reports indicate elevated proton conductivity in near-surface water, though the magnitude and mechanism are debated. Measurements show perpendicular dielectric response within several layers drops to ε⊥ ≈ 2, while in-plane dielectric properties remain unknown.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]