"Enzymatic inhibitors are indispensable tools for dissecting biological pathways and developing therapeutic interventions1. They are broadly categorized by their binding sites and mechanisms of action. Among these, orthosteric inhibitors, which bind to the catalytic site and directly compete with substrates, have been extensively explored due to their predictable structure-activity relationships. However, such inhibitors are typically substrate-agnostic, as their mechanism relies solely on blocking the active site."
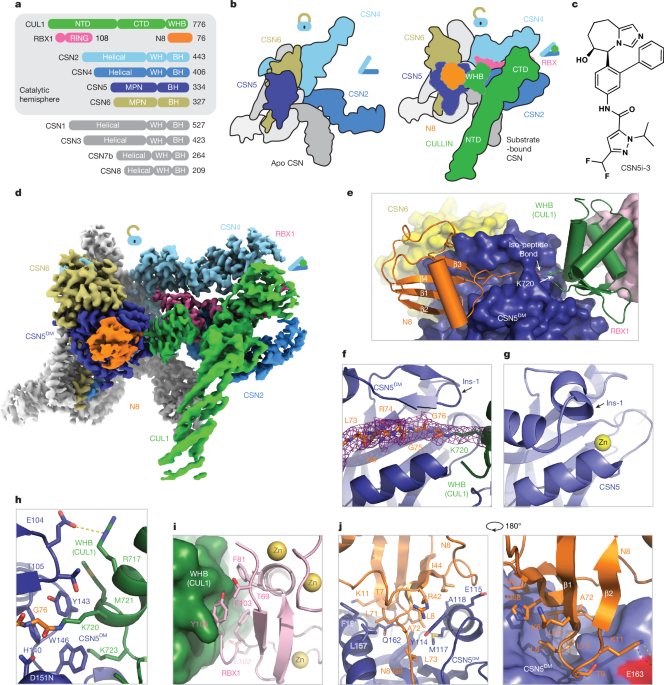
"As the largest family of E3s, the function of CRLs hinges on the dynamic modification of the cullin scaffolds by a ubiquitin-like protein, NEDD8 (N8), also known as neddylation13. Although cullin neddylation enhances the E3 activity of CRLs, CSN-mediated cullin deneddylation is thought to protect the CRL substrate receptors from auto-ubiquitination and promote their exchange on the cullin scaffolds through an adaptive CRL assembly cycle14,15."
Orthosteric inhibitors bind catalytic sites and directly compete with substrates, offering predictable structure-activity relationships but typically acting in a substrate-agnostic manner. Substrate-dependent inhibitors achieve selectivity by engaging allosteric sites or exosites and can modulate enzyme activity in a substrate-specific way, though their design is challenging due to complex structural and dynamic determinants. A hybrid strategy that combines orthosteric tractability with substrate-specific precision could be powerful, yet its feasibility is unresolved. The COP9 signalosome (CSN) is an evolutionarily conserved, eight-subunit complex that regulates cullin-RING ligases (CRLs) and includes the catalytic subunit CSN5.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]