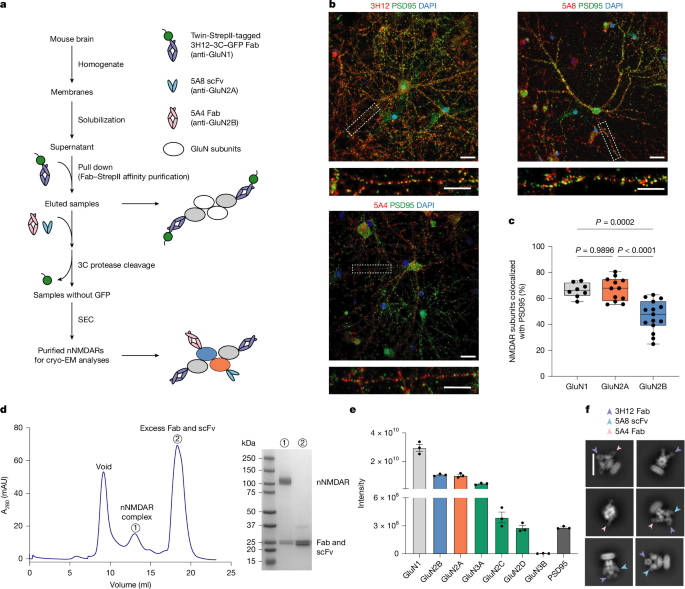
"N-methyl-d-aspartate receptors (NMDARs) are glutamate-gated ion channels that mediate excitatory neurotransmission throughout the brain1. As obligate heterotetramers, their activation requires the binding of both glycine and glutamate2. Although recent structural studies have provided insights into endogenous receptors from select brain regions3, most previous work has relied on recombinant receptors and engineered constructs, which limits our understanding of native NMDARs across the whole brain."
"Here we identify and resolve ten distinct native NMDAR assemblies from the whole-brain tissue of female C57BL/6 mice using immunoaffinity purification, single-molecule total internal reflection fluorescence microscopy and cryo-electron microscopy. Analyses of the GluN1GluN2A(S1), GluN1GluN2A(S2), GluN1GluN2A(S3), GluN1GluN2B, GluN1GluN2AGluN2B(S1), GluN1GluN2AGluN2B(S2), GluN1GluN2AGluNX(S1), GluN1GluN2AGluNX(S2), GluN1GluN2BGluNX and GluN1GluNX structures reveal that GluN2A is the most prevalent subunit across assemblies."
"Moreover, the substantial conformational flexibility observed in the GluN2A amino-terminal domain may explain its fast kinetics and dominant role in gating. Dynamic movements of S-ketamine were also captured at the channel vestibule, as was pore dilation in both the GluN1 and GluN2B subunits of a native GluN1GluN2B receptor."
NMDARs were isolated from whole-brain tissue of female C57BL/6 mice using immunoaffinity purification, single-molecule total internal reflection fluorescence microscopy and cryo-electron microscopy. Ten distinct native NMDAR assemblies were identified and structurally resolved, including multiple GluN1GluN2A variants, GluN1GluN2B, and mixed GluN2A/GluN2B or GluNX configurations. GluN2A is the most prevalent subunit across assemblies. The GluN2A amino-terminal domain exhibits substantial conformational flexibility that may underlie fast kinetics and a dominant role in gating. Dynamic movements of S-ketamine were observed at the channel vestibule, and pore dilation was detected in GluN1 and GluN2B subunits of a native GluN1GluN2B receptor.
Read at www.nature.com
Unable to calculate read time
Collection
[
|
...
]