"The first MOF Robson built used copper as its metal of choice. It was linked to an organic molecule that retained its rigid structure through the presence of a benzene ring, which doesn't bend. Both the organic molecule and the copper could form four different bonds, allowing the structure to grow by doing the rough equivalent of stacking a bunch of three-sided pyramids-a conscious choice by Robson."
"In this case, however, the internal cavities remained filled by the solvent in which the MOF was formed. But the solvent could move freely through the material. Still, based on this example, Robson predicted many of the properties that have since been engineered into different MOFs: the ability to retain their structure even after solvents are removed, the presence of catalytic sites, and the ability of MOFs to act as filters."
"All of that might seem a very optimistic take for someone's first effort. But the measure of Robson's success is that he convinced other chemists of the potential. One was Susumu Kitagawa of Kyoto University. Kitagawa and his collaborators built a MOF that had large internal channels that extended the entire length of the material. Made in a watery solution, the MOF could be dried out and have gas flow through it, with the structure retaining molecules like oxygen, nitrogen, and methane."
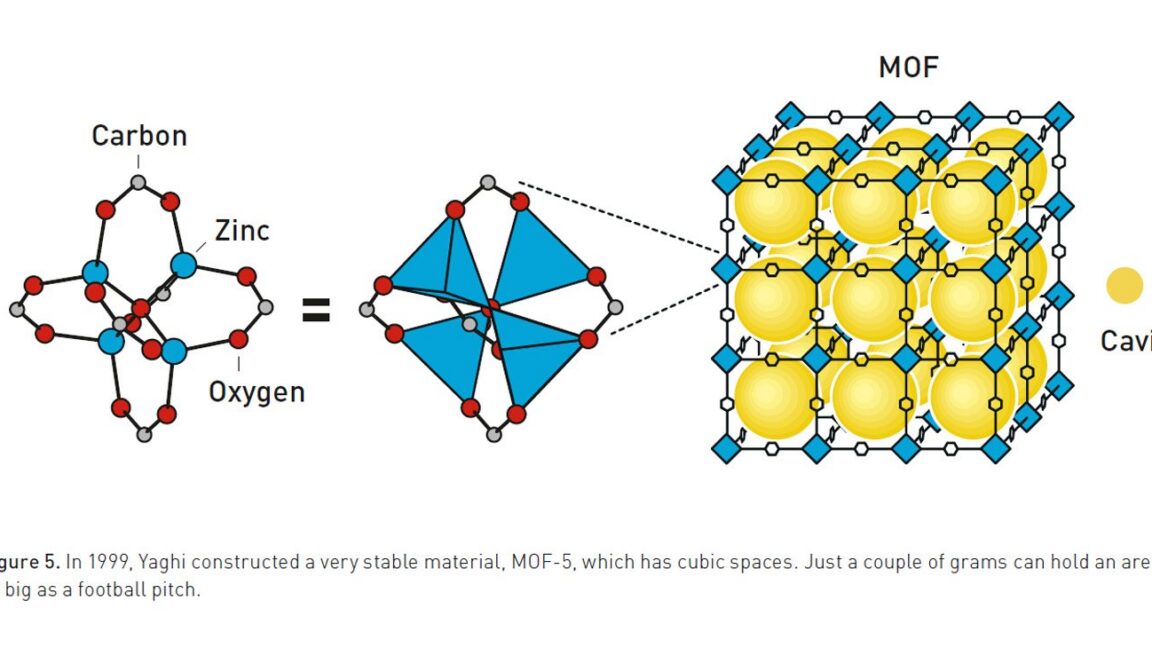
Metal-organic frameworks (MOFs) combine metal centers and rigid organic linkers to form porous, crystalline structures with well-defined internal cavities. The pores can selectively admit or exclude molecules based on size while metal sites offer catalytic activity or selective binding within mixtures. Early skepticism predicted collapse of such open frameworks, but experimental examples demonstrated structural stability even when solvents were removed. The first MOF used copper linked to a benzene-containing organic ligand, forming a network by fourfold coordination that produced three-sided pyramid-like motifs. Subsequent designs produced extended channels retaining gases like oxygen, nitrogen, and methane, enabling filtration and gas storage applications.
Read at Ars Technica
Unable to calculate read time
Collection
[
|
...
]