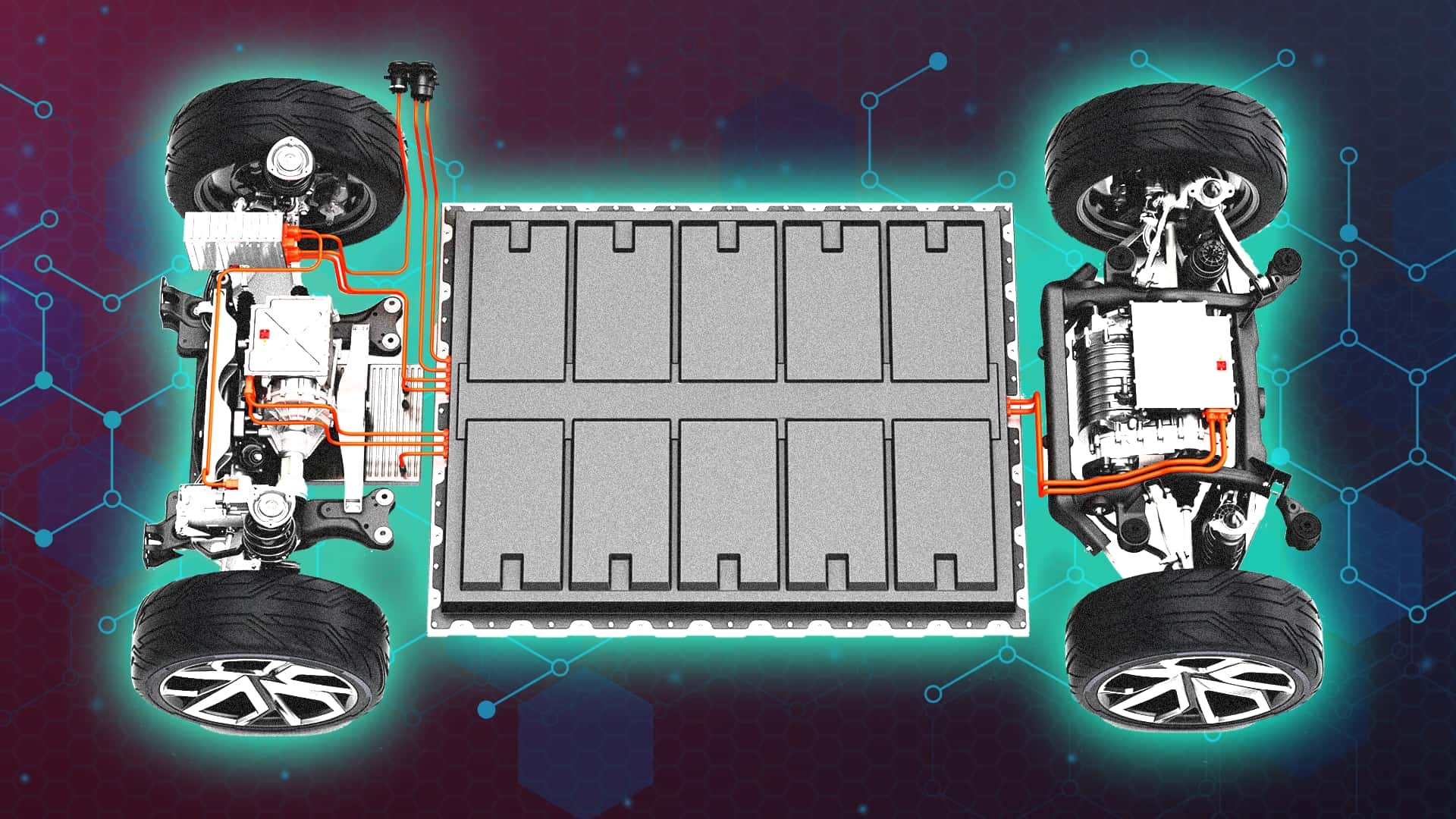
"There's no one-size-fits-all solution when it comes to batteries, especially those used in automotive applications. For electric vehicles, automakers use different chemistries, cell formats and pack designs based on trade-offs around cost, range and performancemuch like how engines range from small naturally aspirated four pots to turbocharged V8s. But if you're a casual EV enthusiast or just someone interested in this space, how do you make sense of it all?"
"Lead-acid batteries are the oldest rechargeable batteries still in widespread use. They're cheap, reliable and easy to recycle. That 12-volt battery in your gas car and your EV? That's a lead-acid battery, as has been the case for decades. However, they're heavy, nowhere as energy-dense as lithium-ion batteries, which is why they tend to be poorly suited for modern EVs. Today, they're mostly used for starter batteries in gas cars for less-demanding auxiliary functions like cabin lights, power windows and infotainment screens."
Automakers choose EV battery chemistries, cell formats and pack designs to balance cost, driving range and performance trade-offs. Lead-acid batteries remain the oldest rechargeable type, offering low cost, reliability and recyclability but poor energy density and heavy weight, so they are used mainly for 12-volt starter and auxiliary functions. Early GM EV1 initially used lead-acid cells before switching to nickel-metal hydride. Nickel-metal hydride batteries are durable and climate-forgiving, commonly used in hybrids, especially Toyota models, but face similar weight and energy-density limitations and are gradually being replaced by lithium-ion packs.
Read at insideevs.com
Unable to calculate read time
Collection
[
|
...
]