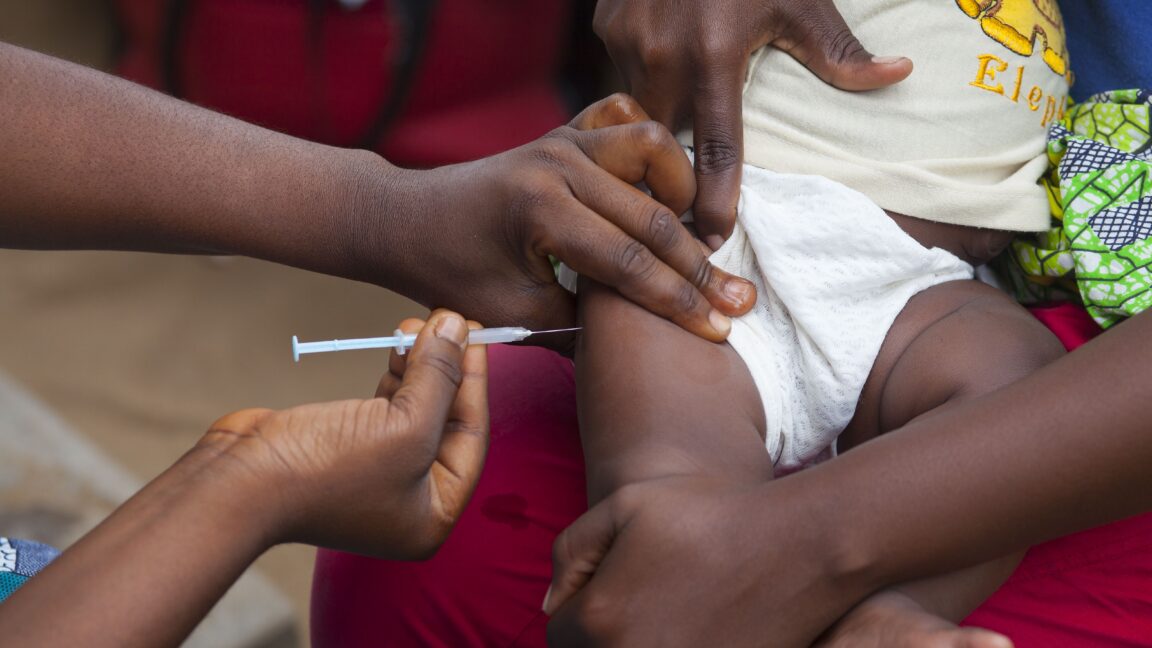
"The United Nations health agency highlighted that the hepatitis B vaccine birth dose is "an effective, and essential public health intervention" that has "been used for over three decades, with more than 115 countries including it in their national schedules." "It prevents life‑threatening liver disease by stopping mother‑to‑child transmission at birth," the WHO wrote, noting that more than 12 percent of adults in Guinea-Bissau have chronic hepatitis B."
""From what is publicly described, the [trial] protocol does not appear to ensure even a minimum level of harm reduction and benefit to the study participants (e.g., screening pregnant women and vaccinating newborns exposed to hepatitis B)," the WHO wrote. "As a proven lifesaving vaccine, withholding it from some study participants would expose newborns to serious and potentially irreversible harm, including chronic infection, cirrhosis, and liver cancer, the WHO argues."
"The WHO also noted that the publicly available information about the trial indicates that it will be a single-blind, no-treatment-controlled design, which "raises a significant likelihood of substantial risk of bias, limiting interpretability of the study results and their policy relevance." As of now, the trial appears to be suspended. Nature News reported that in a January 22 press conference, health officials in Guinea-Bissau said that a technical and ethical review was pending."
The WHO states that the hepatitis B vaccine birth dose is an effective, essential public health intervention used for over three decades and included in more than 115 national schedules. The birth dose prevents life‑threatening liver disease by stopping mother‑to‑child transmission; Guinea‑Bissau has over 12 percent adult chronic hepatitis B prevalence. The WHO finds the trial protocol does not ensure minimum harm reduction such as screening pregnant women or vaccinating exposed newborns. Withholding the vaccine would risk chronic infection, cirrhosis, and liver cancer. The trial's single‑blind, no‑treatment design risks bias and reduces policy relevance. The trial appears suspended pending a technical and ethical review.
Read at Ars Technica
Unable to calculate read time
Collection
[
|
...
]