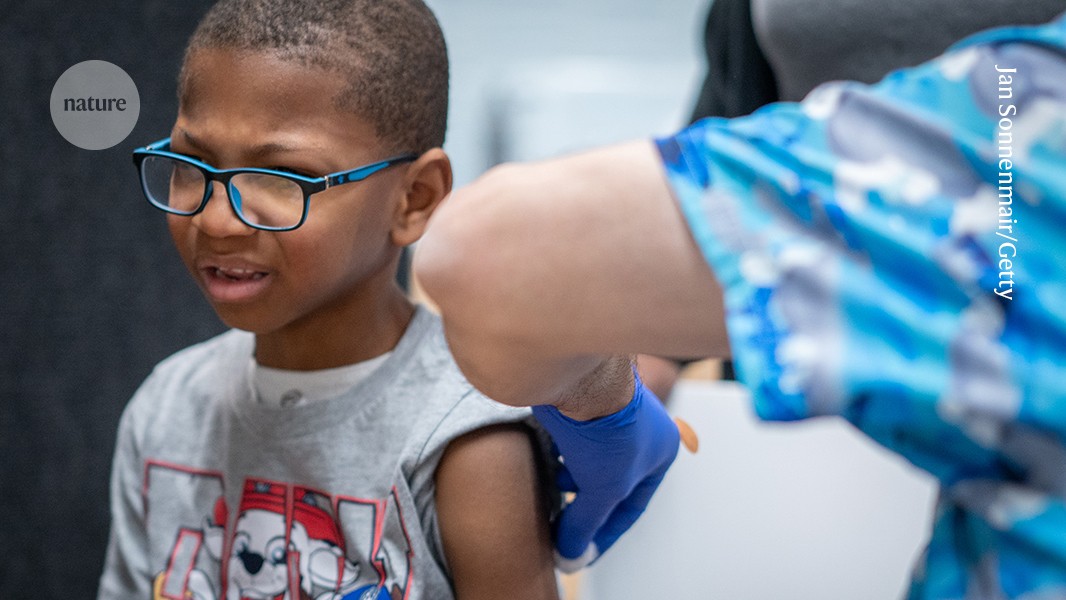
"Next week, the panel of top advisers who recommend how vaccines are used in the United States will meet to review jabs that protect against COVID-19, hepatitis B and other diseases. The meeting - the committee's second since US health secretary and anti-vaccine advocate Robert F. Kennedy Jr abruptly fired all of its previous 17 members and welcomed 7 new ones - has raised eyebrows among public-health specialists, given that the safety and efficacy of some of the vaccines on the agenda have been well established"
""I'm very concerned, given the signalling from the members of this newly reconstituted, hand-picked [committee] that they are going to select targets for further restriction," says Andrew Pavia, a physician with the Infectious Diseases Society of America, a medical association based in Arlington, Virginia. At their last meeting, the advisers - several of whom have publicly expressed anti-vaccine views - voted to end the use of the preservative thimerosal in influenza vaccines despite evidence that it is safe at the doses found in jabs."
Next week's Advisory Committee on Immunization Practices meeting will review vaccines for COVID-19, hepatitis B and other diseases. The meeting is the second since US health secretary Robert F. Kennedy Jr fired the committee's previous 17 members and appointed seven new members. Public-health specialists express concern because some vaccines under review have long-established safety and efficacy. Several newly appointed advisers have publicly expressed anti-vaccine views and previously voted to end thimerosal use in influenza vaccines despite evidence of safety at the doses used. The agenda lacks detail and was posted with only a month's notice, raising worries about restricted deliberation.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]