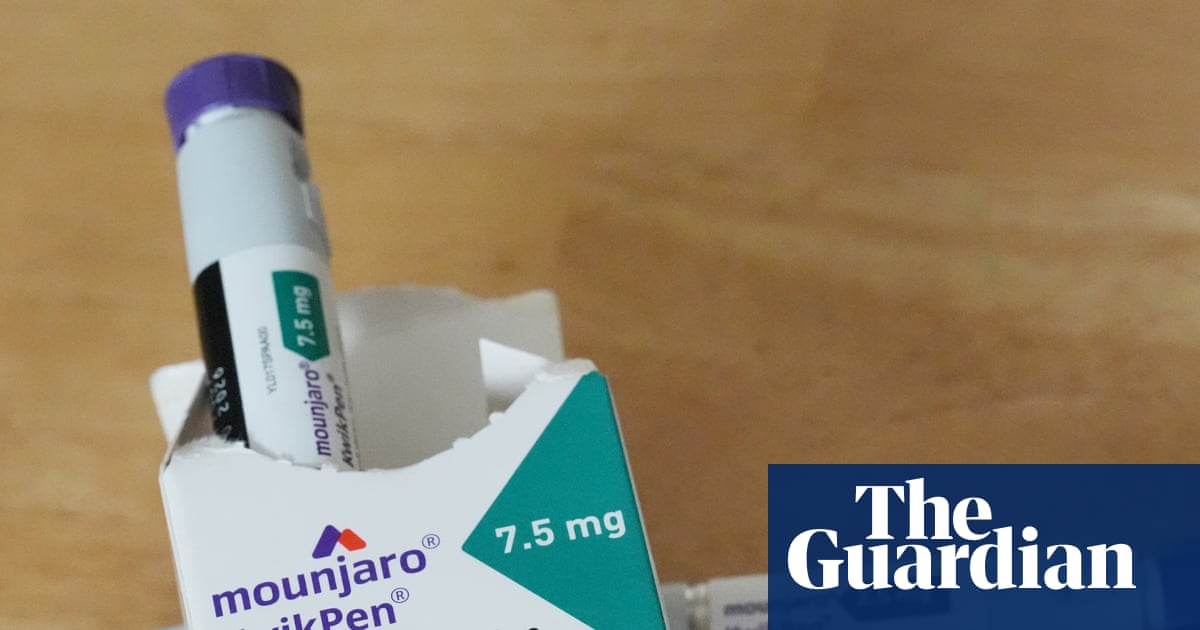
"Retatrutide, which is unlicensed in the UK, is an experimental injection developed by the US drugmaker Eli Lilly. Tirzepatide, more commonly known under its brand name Mounjaro, is available on the the NHS for weight loss for patients who fulfil a certain criteria of comorbidities. The site, on an industrial estate on the outskirts of Northampton, is believed to have been used for the large-scale manufacture, packaging and distribution of unlicensed and potentially deadly weight-loss products to customers."
"These unregulated products, made with no regard for safety or quality, posed a major risk to unwitting customers. My message is clear: don't buy weight-loss medications from unregulated sources. Talk to your GP, seek NHS advice, and don't line the pockets of criminals who don't care about your health. Safe, appropriate, licensed obesity drugs can greatly benefit those in need if taken under medical supervision, and I urge people to only purchase and use them with the approval and oversight of medics and pharmacists."
The Medicines and Healthcare products Regulatory Agency, supported by Northamptonshire police, raided and dismantled a factory on an industrial estate in Northampton after a two-day search. Tens of thousands of empty weight-loss pens, raw chemical ingredients, and more than 2,000 unlicensed retatrutide and tirzepatide pens were seized. Retatrutide is experimental and unlicensed in the UK; tirzepatide (Mounjaro) is available on the NHS for certain eligible patients. The finished products have an estimated street value exceeding £250,000. The operation is part of ongoing enforcement against the illegal trade in weight-loss medicines, and officials warned consumers to only use licensed drugs under medical supervision.
Read at www.theguardian.com
Unable to calculate read time
Collection
[
|
...
]