"But questions remain about the accuracy and uncertainty of these tests, and experts caution that the assays aren't ready for prime time. While the results here are encouraging, they are not yet at the level of having significant clinical benefit for individual patients, says Corey Bolton, a clinical neuropsychologist and an assistant professor of medicine at Vanderbilt University Medical Center, who was not involved in the new study."
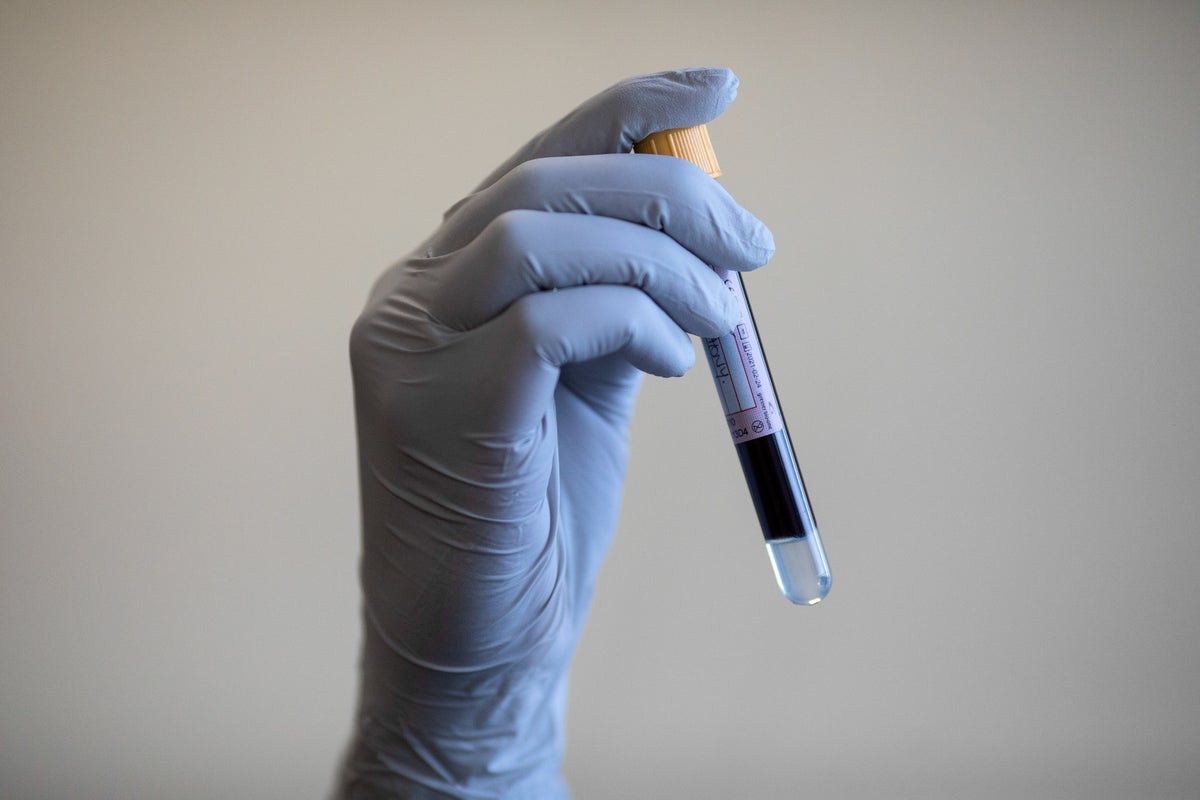
"Alzheimer's disease is a complex condition with numerous intersecting risk and resilience factors that vary from person to person. These factors can have a large influence in the age of symptom onset and the rate of clinical decline. The study included more than 600 people aged 62 to 78 who were not cognitively impaired. They had blood tests to detect a protein called p-tau217, which accumulates in the brains of people with Alzheimer's."
More than 600 cognitively unimpaired people aged 62 to 78 underwent blood tests measuring p-tau217, a protein that accumulates in Alzheimer's brains. A model using those results estimated each person’s likely age of symptom onset with about three to four years of uncertainty. The assays show promise for early prediction but currently lack enough accuracy and certainty for meaningful clinical decisions for individuals. Alzheimer's disease involves many intersecting risk and resilience factors that vary between people and substantially influence onset age and rate of clinical decline. Several people connected to the work have consulting or funding ties to companies making these tests.
Read at www.scientificamerican.com
Unable to calculate read time
Collection
[
|
...
]