"Lipid peroxidation involves the formation of lipid and lipid peroxyl radicals, which, in auto-oxidative propagation reactions, generate lipid hydroperoxides. Aberrant, unrestricted lipid peroxidation results in altered membrane integrity, cell swelling, and ultimately membrane rupture11,12. In recent years, growing interest in ferroptosis has revealed that cells depend on several enzymes to protect against ferroptosis6,7,8,9,13,14,15, of which GPX4 and FSP1 have key roles."
"GPX4 catalyses the reduction of PUFA-PL hydroperoxides to non-toxic alcohols. FSP1 catalyses the reduction of extramitochondrial coenzyme Q 10 (CoQ), a highly potent lipid radical-trapping antioxidant (RTA). Conversely, cells can be sensitized to ferroptosis through PUFA-PL composition16 and biosynthesis by ACSL417,18,19. In addition to these cellular pathways, exogenous lipid RTAs, such as LIP1, FER1, vitamin E and vitamin K, have also been shown to specifically inhibit ferroptosis1,6,20,21,22."
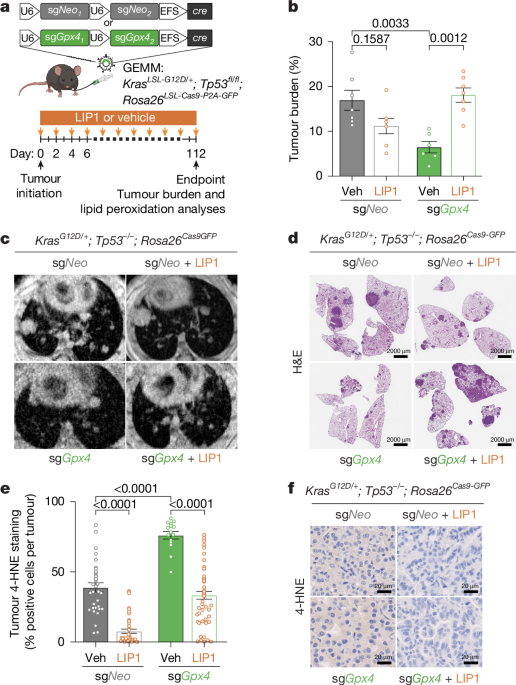
Ferroptosis is a regulated, non-apoptotic cell death driven by iron-dependent lipid peroxidation of polyunsaturated membrane phospholipids, producing lipid hydroperoxides that compromise membrane integrity and cause cell rupture. Enzymatic defenses limit ferroptosis, notably GPX4, which reduces PUFA-PL hydroperoxides to alcohols, and FSP1, which reduces extramitochondrial CoQ to a lipid radical-trapping antioxidant. PUFA-PL composition and ACSL4-dependent biosynthesis sensitize cells to ferroptosis. Exogenous lipid radical-trapping antioxidants such as LIP1, FER1, vitamin E and vitamin K can inhibit ferroptosis. Cancer cells, including drug-resistant mesenchymal variants, show heightened sensitivity to lipid peroxidation, suggesting therapeutic potential, while in vivo roles of GPX4 and FSP1 remain incompletely characterized.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]