"Influenza A viruses are enzootic in wild migratory birds of aquatic habitats around the world7. In wild waterfowl, 17 subtypes of HA (H1-H16, H19) and 9 of neuraminidase (NA, N1-N9), surface glycoproteins of influenza A viruses, have been identified8,9. HA is the receptor-binding and fusion protein of influenza A viruses, and its head domain contains dominant epitopes targeted by antibodies."
"A(H5) HPAIVs from the A/goose/Guangdong/1/1996 (GsGd) lineage were first detected in Hong-Kong in 199710,11, and have since spread globally. This lineage is now enzootic in poultry and wild birds in many regions1. A(H5) GsGd HPAIVs have infected over 60 mammalian species2, caused mass die-offs in marine mammals and widespread outbreaks in US dairy cows14, leading to substantial animal health and economic impacts."
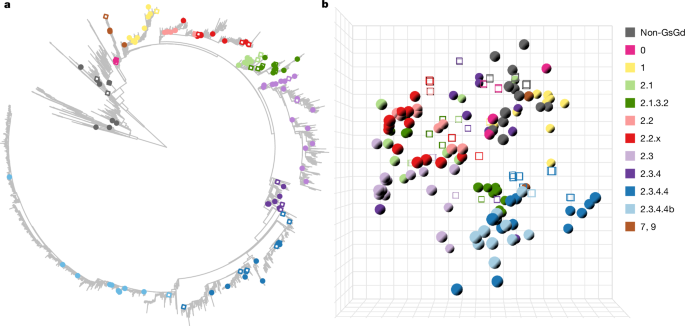
Wild migratory aquatic birds act as enzootic reservoirs for Influenza A viruses, with 17 HA and 9 NA subtypes identified in wild waterfowl. HA functions as the receptor-binding and fusion protein, and its head domain contains dominant epitopes targeted by antibodies. Avian and human influenza viruses preferentially bind α2,3- and α2,6-linked sialic acid receptors, respectively, and receptor specificity is a major host range determinant. A(H5) and A(H7) subtypes can evolve into highly pathogenic avian influenza viruses in poultry. The A(H5) GsGd lineage emerged in 1997, became globally spread and enzootic in many regions, infected diverse mammals, caused severe animal outbreaks, spilled over to humans with substantial fatalities, and has undergone antigenic diversification prompting frequent WHO candidate vaccine updates.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]