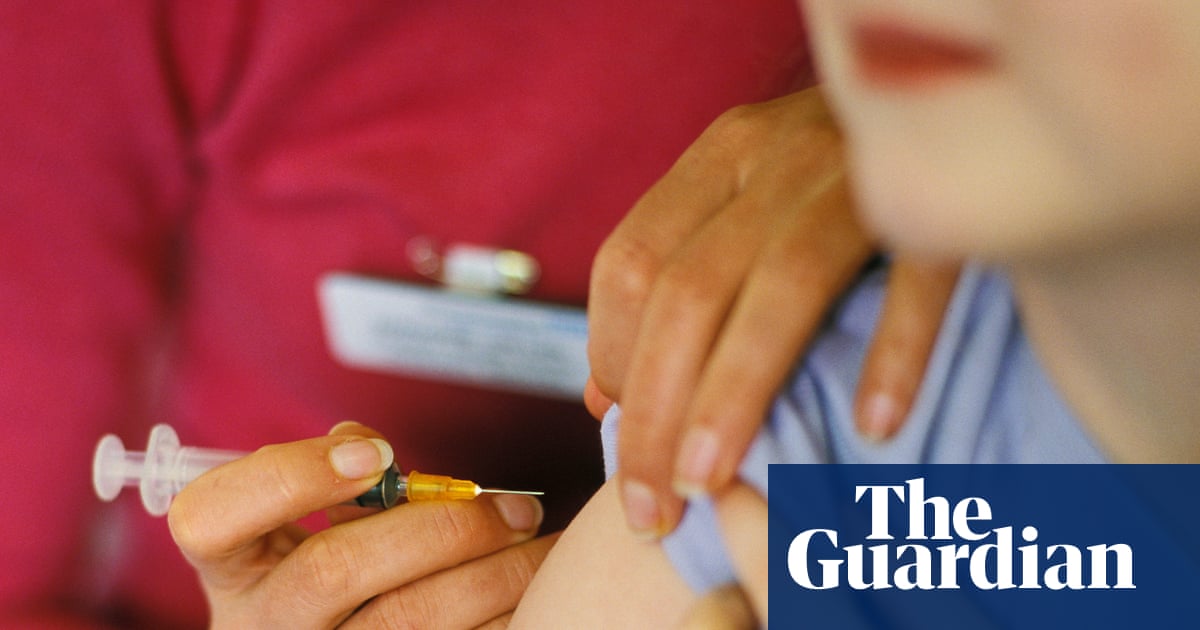
"However, the 2024 Cass review of NHS gender identity services for children and young people found there was insufficient/inconsistent evidence about the effects of puberty suppression on psychological or psychosocial wellbeing, cognitive development, cardio-metabolic risk or fertility. NHS England subsequently announced children with gender dysphoria would no longer receive puberty blockers as routine practice, with their use confined to research settings."
"The youngest participants are expected to be 10 to 11 for biological females and 11 to 12 for biological males although the team note the rigorous selection process means participants will probably be older and the upper age limit for joining the study is 15 years and 11 months. Participants will be randomised to immediately start puberty blockers or begin using the drugs after a year's delay, alongside a wider package of care and support."
Two new UK studies will investigate the impact of puberty blockers in young people with gender incongruence. Puberty blockers were originally used to treat early-onset puberty and have been used off-label for children with gender dysphoria or incongruence. The 2024 Cass review found insufficient or inconsistent evidence on effects of puberty suppression on psychological or psychosocial wellbeing, cognitive development, cardio-metabolic risk, or fertility. NHS England restricted routine use of puberty blockers in children with gender dysphoria to research settings. The Pathways programme includes the Pathways Trial, aiming to recruit about 226 participants aged roughly 10–15, randomised to immediate or delayed treatment and monitored for 24 months, with individual clinical reviews and comparisons to another group.
Read at www.theguardian.com
Unable to calculate read time
Collection
[
|
...
]