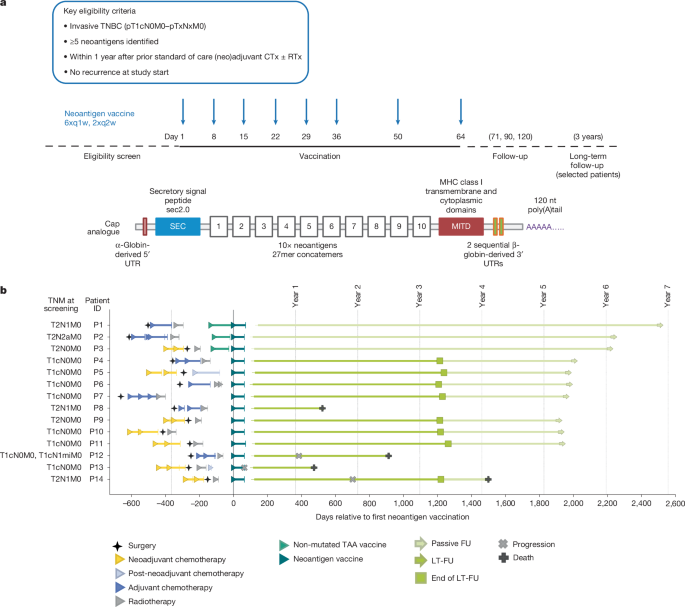
"DNA repair deficiency, intrinsic genomic instability and an immunogenic tumour microenvironment make TNBC an interesting candidate for individualized vaccination with somatic cancer mutations that can act as neoantigens2,3. We developed an individualized neoantigen RNA vaccine approach that uses next generation sequencing (NGS) for mutation calling and predicts potentially T cell immunity-inducing neoantigens. This approach is being studied in patients with melanoma and pancreatic and other solid cancers4,5,6,7 (Extended Data Fig. 1a)."
"To create the vaccine, multiple cancer mutations from an individual patient are concatemerized and encoded on two strings of immunostimulatory, non-nucleoside-modified uridine mRNA. Molecular designs of the cap, 5′ and 3′ untranslated regions, and the poly(A) tail of the mRNA enhance translation of the coding sequence in human dendritic cells8,9. A secretion signal and an MHC class I trafficking domain (MITD) tag are fused to the coding sequence to augment human leukocyte antigen (HLA) class I and II presentation of the encoded antigen and recognition by CD8+ and CD4+ T cells10."
"Formulation in liposomal nanoparticles (LPXs) for intravenous administration targets resident dendritic cells in all lymphoid compartments of the body11,12 (Fig. 1a). The use of uridine mRNA in the RNA-LPX vaccine technology links antigen delivery with co-stimulation via Toll-like receptor (TLR)-mediated, type-I-interferon-driven antiviral immune responses, resulting in profound expansion of antigen-specific T cells in humans, which is effective even against non-mutant self-antigens."
TNBC tends to recur and metastasize even at early disease stages, with recurrence rates peaking around three years and declining rapidly thereafter. DNA repair deficiency, intrinsic genomic instability and an immunogenic tumour microenvironment create susceptibility to neoantigen-targeted vaccination. An individualized neoantigen RNA vaccine workflow uses next-generation sequencing for mutation calling and prediction of T cell immunity-inducing neoantigens. Multiple somatic mutations are concatemerized and encoded on two strands of non-nucleoside-modified uridine mRNA with optimized cap, untranslated regions and poly(A) tail to enhance translation in human dendritic cells. A secretion signal and an MITD tag augment HLA class I and II presentation, and liposomal nanoparticle formulation delivers RNA-LPX intravenously to resident dendritic cells while uridine mRNA engages TLR-mediated type I interferon responses to expand antigen-specific T cells.
Read at Nature
Unable to calculate read time
Collection
[
|
...
]